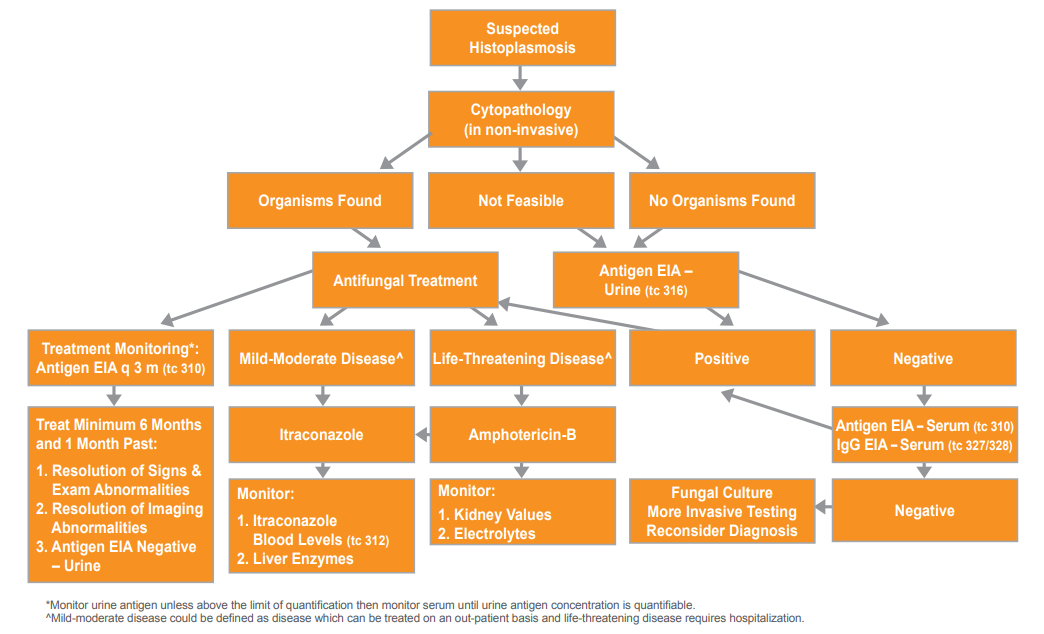
Diagnostic and Treatment Algorithm for Histoplasmosis in Dogs or Cats
* Monitor urine antigen unless above the limit of quantification then monitor serum until urine antigen concentration is quantifiable.
^ Mild-moderate disease could be defined as disease which can be treated on an out-patient basis and life-threatening disease requires hospitalization.