Antigen Testing Guides Individualized Medical Care ─ Treatment Monitoring for Histoplasmosis and Blastomycosis
Case Example
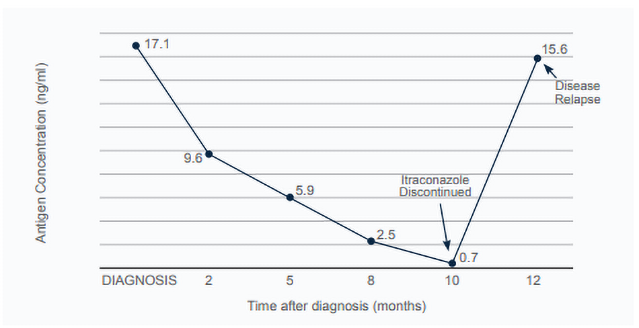
Boone is a 3-year-old, male, mixed breed dog that was diagnosed with disseminated histoplasmosis. Histoplasma yeasts were found on a blood smear (fungemia). Boone’s presenting signs included mixed bowel diarrhea, weight loss, lethargy, and anorexia. Physical examination revealed a fever (103.8F), poor body condition (2/9), abdominal organomegaly, and peripheral lymphadenopathy. Boone was started on FDA generic itraconazole capsules (6 mg/kg/day PO). MVista® Histoplasma Antigen Quantitative EIA on urine was positive (17.1 ng/mL). Boone responded well to treatment and over the following 2 months his clinical signs significantly improved. For treatment monitoring, urine antigen concentrations were measured by the MVista® Histoplasma Antigen Quantitative EIA, every 2-3 months. Antigen concentrations decreased over time (Figure 1). After 8 months of treatment, Boone was reportedly back to normal. Against the attending veterinarian’s advice, after 10 months of treatment, the pet-owners discontinued Boone’s itraconazole. Urine antigen concentration at that time was 0.7 ng/mL. Approximately 2 months later, Boone was represented for fever, anorexia, and lethargy. Urine Histoplasma Antigen concentration at that time had significantly increased from previous visit (15.6 ng/mL). Itraconazole was again prescribed and Boone again responded positively.
Figure 1: Boone’s urine Histoplasma antigen concentrations over time
Discussion
Boone’s case is an example of how urine antigen concentrations can be used to help guide individualized antifungal treatment. It is common for clinical signs to resolve before the fungus is completely eliminated. Studies have shown, that for histoplasmosis and blastomycosis, antigen concentrations decrease with successful treatment [1
Recommendations include treating until the urine antigen concentration is negative. Ideally all of the following criteria are met before discontinuing antifungal treatment for histoplasmosis or blastomycosis. Less commonly, when all other criteria are met, antifungal therapy can be successfully discontinued when urine antigen concentrations are very low (positive but below the limit of quantification, BLQ. Early on, if Histoplasma urine antigen concentrations are extremely high, and not quantifiable (above the limit of quantification, ALQ), serum antigen concentrations can be used instead for monitoring.
- Minimum of 6 months of antifungal treatment
- Resolution of clinical signs
- Resolution of diagnostic imaging abnormalities (or minimal static disease)
- Negative urine antigen test
Disease relapse can be demoralizing for the pet-owner and places the pet at risk for euthanasia. Ideally disease relapse is avoided, but when it does occur, early detection is essential. Studies have shown that urine antigen concentrations increase with disease relapse [1
In addition to antigen concentration monitoring, itraconazole blood levels should be monitored. The absorption of itraconazole in dogs and cats is highly variable and a starting dose is only a starting point [3
REFERENCES:
- Hanzlicek AS, Meinkoth JH, Renschler JS, et al. Antigen Concentrations as an Indicator of Clinical Remission and Disease Relapse in Cats with Histoplasmosis. J VER INTERN MED 2016;30:1065-1073.
- Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J VET INTERN MED 2014;28:305-310.
- Renschler J, Albers A, Sinclair-Mackling H, Wheat LJ. Comparison of compounded, generic, and innovator-formulated itraconazole in dogs and cats. J AM ANIM HOSP ASSOC 2018;54:195-200.