What Tests Should Be Performed for Diagnosing Coccidioidomycosis?
Drew Hanzlicek, DVM, MS, DACVIM (SAIM); Joe Wheat, MD; Heather Largura, DDS, MS
Diagnosis
The most sensitive testing option for coccidioidomycosis is a combination of tests for antibodies including the MiraVista IgG EIA (MVista® Cocci IgG) and Immunodiffusion (Cocci ID). This combination provides a sensitivity of >98%, while still being highly specific (97%). An alternate combination, also with high sensitivity (>93%), includes the combination of testing for antibody with the MVista® Cocci IgG and for antigen with the MiraVista Coccidioides urine antigen EIA (MVista® Cocci Ag). Antigen testing alone lacks sensitivity, but when positive can be used to guide treatment monitoring as can antibody testing.
| Test(s) | Sensitivity (%) | Test Code(s) | Reference |
|---|---|---|---|
| MVista® Cocci Ab IgG | 89.2 | 315 | Holbrook et al. 2019 |
| MVista® Cocci Ab IgG + Cocci Ab ID | 98.6 | 329 + 320 | Holbrook et al. 2019 |
| MVista® Cocci Ab IgG + MVista Cocci Ag EIA | 93.2 | 329 + 315 | Holbrook et al. 2019 |
Ab = antibody; ID = immunodiffusion; EIA = enzyme immunoassay
Monitoring Treatment
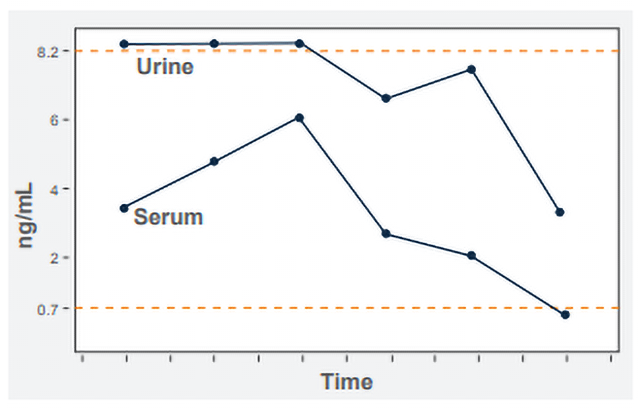
Antigen is quantified by extrapolation from a standard curve containing standards ranging from 0.07 ng/mL to 8.2 ng/mL. Results above the highest standard are classified as above the limit of quantification (ALQ). The reason for not providing the ng/mL concentration is that the standard curve is no longer linear above the highest standard. The drawback of an ALQ result is that response cannot be monitored by antigen clearance if the ALQ result persists. If antibody is detected, we recommend also testing urine and serum for antigen to provide the best marker for monitoring response to treatment and diagnosing relapse. Monitoring the serum usually permits quantification when the urine is ALQ (Figure).
When antigen testing is negative, antibody testing can be used to help guide treatment, although a specific cut-off for when to discontinue treatment, has not been established. Intuitively, the MVista® Cocci IgG or Cocci ID should be negative or a very low positive before stopping treatment. The MVista® Cocci IgG is fully quantitative which should facilitate treatment monitoring as compared with Cocci ID titers.
We Recommend
- Send both urine and serum for diagnosis of coccidioidomycosis
- Test serum for antibodies with MVista® Cocci IgG and Cocci ID
- Test the urine and serum for MVista® Cocci antigen if:
- The antibody is negative but coccidioidomycosis is suspected
- The antibody is positive to provide a quantifiable marker for treatment monitoring
- Continue to monitor the serum for antigen if the urine antigen is ALQ.
- Revert to testing urine once serum antigen is negative
- Monitor MVista® Cocci IgG antibody or Cocci ID antibody if the MVista® Cocci antigen is negative. The IgG antibody, if positive, is more quantifiable than titration of ID antibody and is preferred over ID. There is no published data on the usefulness of monitoring antibody as a marker for when to stop therapy, and antigen is a marker for infection while antibody is a measure to the immune response, not a marker for infection.
- 3-month intervals during treatment
- 6 and 12 months after stopping treatment
- If the clinical or imaging findings suggest progression or relapse
- Criteria for stopping treatment:
-
- Clinical findings have resolved
- Imaging abnormalities have resolved or have minimal static changes
- Antigen in urine and serum is negative (if positive initially)
- Antibody is negative or very low positive (if positive initially)
- Obtain consultation if weakly positive after 12 months of treatment
REFERENCES:
- Holbrook ED, Greene RT, Rubin SI, et al. Novel canine anti-Coccidioides immunoglobulin G enzyme immunoassay aids in diagnosis of coccidioidomycosis in dogs. Med Mycol 2019;57:800-806.