Andrew Hanzlicek, DVM, MS, DACVIM
At a Glance – Key Points
- Pythiosis can affect dogs and cats anywhere in the U.S.
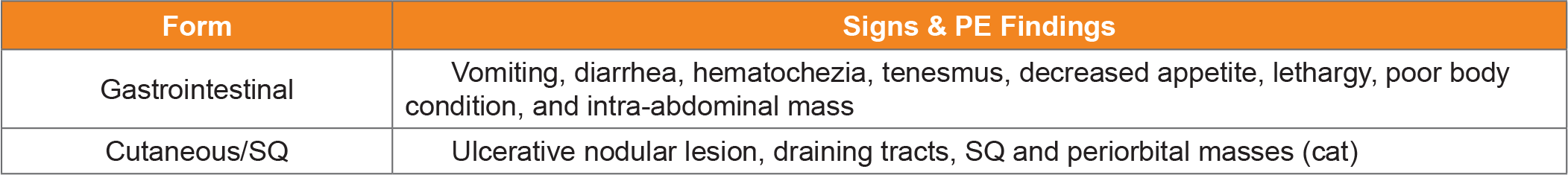
- Cutaneous/SQ and GI disease is most common.
- Hyphal organisms can be absent in cytopathology or histopathology samples, but when found cannot
be differentiated from many pathogenic molds based on morphology alone. - MiraVista Pythium IgG enzyme immunoassay (EIA) is highly specific (>99%) and sensitive (>99%)
for the diagnosis of all forms of pythiosis in dogs and cats. - MiraVista Pythium IgG EIA can be used for treatment monitoring as IgG levels decrease and normalize
with successful treatment.
Introduction
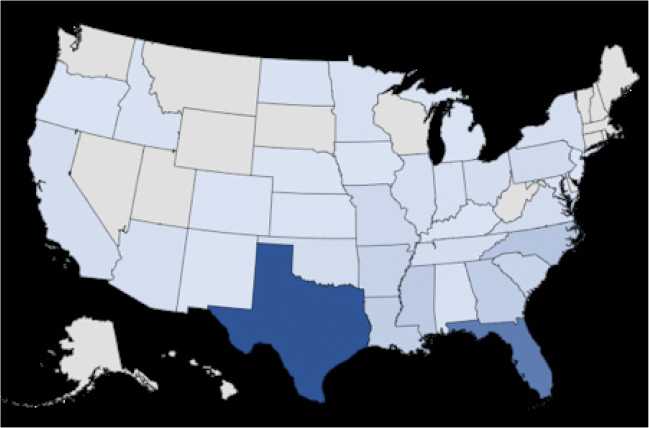
Pythium insidiosum (and cryptic species P. periculosum)1, the causative agent of pythiosis, are a fungal-like pathogens classified as oomycetes. Dogs are more often affected than cats, but any mammal is susceptible. Horses, sheep, and cattle are the most common large animal species affected.2,3 Pythiosis is uncommon in humans, most often causing ocular or vascular disease.2 Pythium is intolerant of cold and is most commonly found in the Southern U.S. – although pythiosis has been diagnosed from CA to AZ to FL to WI and everywhere in between. (Figure 1) There are seasonal trends, with the most dogs being diagnosed in September – February.4
In the environment motile biflagellate zoospores are found in warm water requiring water plants for the lifecycle. It is likely in water where dogs and cats are infected when the organism penetrates skin or is ingested and penetrates the GI tract. Plant ingestion might also be a mode of infection. In tissues it is found as hyphae where it causes a severe inflammatory reaction that alters organ structure and function. The most common sites of infection are skin/SQ and GI tract. Often one or other site is affected, but occasionally both organ systems are involved. Infections tend to be slowly progressive and locally invasive, while dissemination to distant sites such as bone, liver, or lung is uncommon.2 Local lymph nodes are often involved.
Gastrointestinal Disease (Figures 6-8)
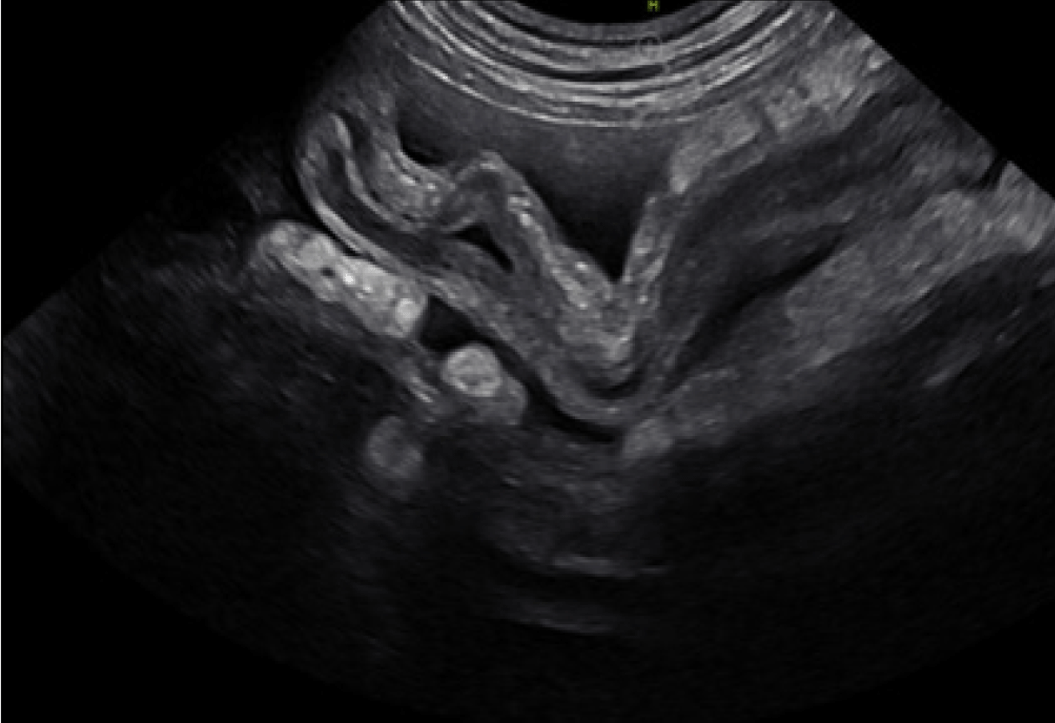
Animals can have GI pythiosis for months, or even years, before showing clinical signs, which can include vomiting, diarrhea, hematochezia, tenesmus, decreased appetite, and weight loss. A palpable abdominal mass might be found on examination. Abdominal ultrasound or CT shows one, or multiple, segmental infiltrative GI masses most commonly at the gastric outflow, duodenum, or ileocolic junction- although disease can be found anywhere from pharynx to rectum.5,6 On ultrasonography a loss of layering often accompanies GI wall thickening.7 Orad luminal dilation suggestive of mechanical obstruction can also be found. Mesenteric lymph nodes are often involved and are enlarged and heterogeneous.7 A mass at the root of the mesentery is another common site and is heterogeneous on ultrasound often including mesenteric lymph nodes. Invasion into the mesenteric vessels can lead to hemorrhage, bowel ischemia, and necrosis, which is invariably fatal.
Cutaneous and Sub-cutaneous Disease (Figures 3-4)
Skin lesions are non-healing, ulcerative and nodular with draining tracts- most often affecting the limbs, ventral trunk, or perineum.5 Cats can have large subcutaneous masses without ulceration or draining tracts most often periorbital, inguinal, or at the tail head.5 Since infection is from cutaneous inoculation, intuitively, traumatic injury of the skin might precede infection, but this is not often noted by pet-owners. Local lymphadenomegaly is common.
Disease of Other Body Systems
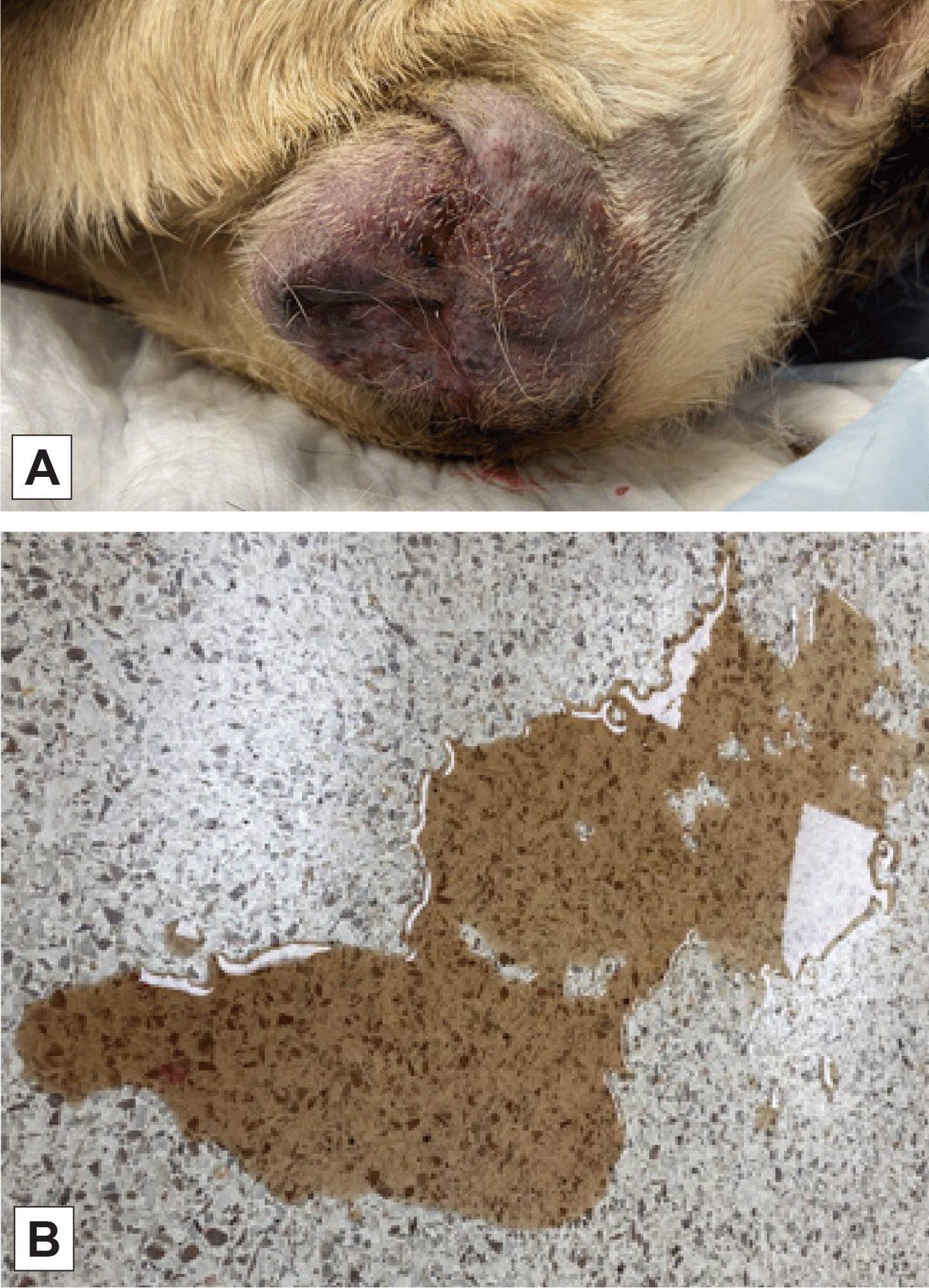
Respiratory involvement causes tachypnea, dyspnea or a cough as tracheobronchial lymphadenopathy compresses large airways rich with cough receptors.8 Urogenital invasion can cause stranguria, pollakiuria, and hematuria. (Figure 5)
Diagnosis
Organisms can be scarce or not present on cytopathology or histopathology. Special stains, such as GMS stain, can help with visualization. Pathology shows mixed pyogranulomatous and eosinophilic inflammation. If found, hyphae are broad (2-7 µm) with irregular branching, sparse septation, and rounded tips (Figure 2).2 Based on morphology alone, these cannot be differentiated from other oomycetes (Lagenidium or Paralagenidium) or hyaline molds including Conidiobolus and Basidiobolus. The organisms are often mistaken for Aspergillus on pathology reports. Routine lab work changes are non-specific and suggestive of chronic inflammation possibly including a mild non-regenerative anemia, eosinophilia, hypoalbuminemia, and hyperglobulinemia.6
Due to diagnostic challenges noted above, a blood-based biomarker to support the diagnosis and assist with treatment monitoring is desirable. MiraVista Diagnostics (MVD) offers an anti- Pythium IgG enzyme immunoassay (EIA) requiring only 0.25 ml serum. Pythium is unique enough from common fungal pathogens (Blasto, Histo, Cocci, Crypto, Asper) that there is no cross-reactivity- making the assay highly specific (>99%). In addition, dogs and cats with pythiosis develop a strong humoral immune response leading to high antibody levels- making the assay highly sensitive (>99%). The MVD Pythium IgG assay is the first commercially available assay that can be used to test dogs and cats. With successful treatment antibody levels drop making the MVD Pythium IgG assay a useful tool for treatment monitoring (see below).
Treatment
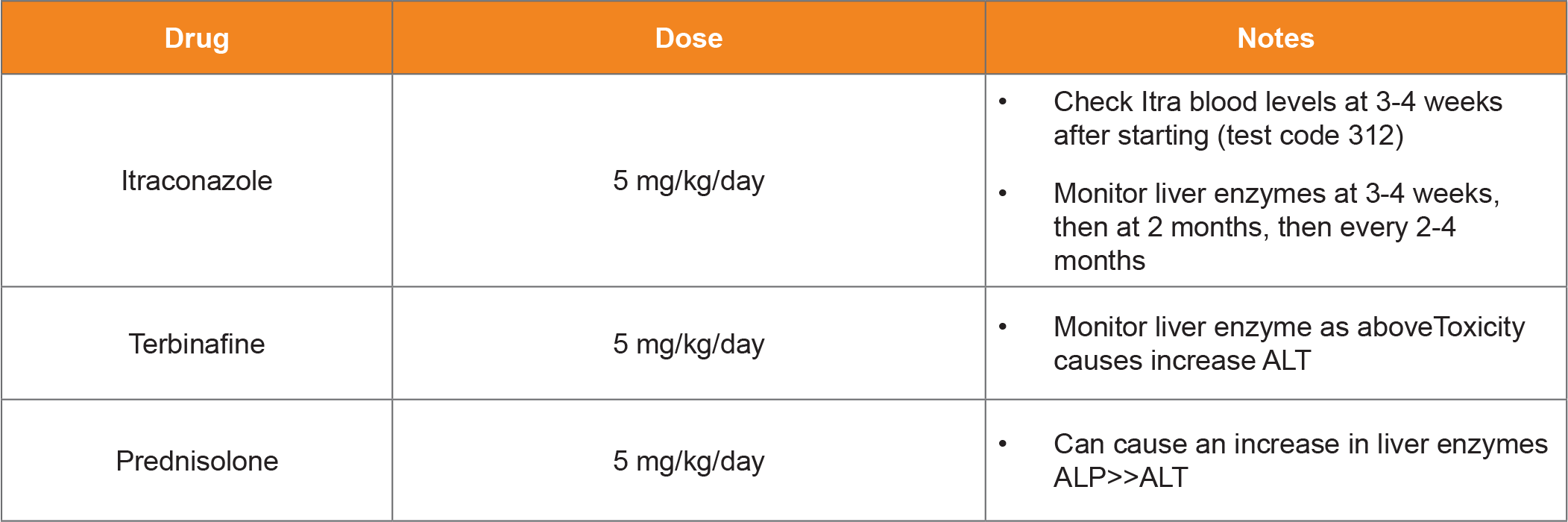
Surgical excision with wide margins is the treatment of choice for cutaneous pythiosis. The surgical approach is similar to that of a soft tissue sarcoma.9 Medical therapy (described below) is recommended for at least 6 months after surgery. Pythium IgG levels should be checked at baseline, monthly for 3 months, then every 3 months until negative after surgery. A persistently positive IgG test is suggestive of either incomplete resection or disease at another site. In the absence of a mechanical GI or bile duct obstruction, medical treatment is an option for GI pythiosis. Itraconazole, terbinafine, and prednisone (Table 2) are combined with a long treatment duration (generally >1 year).10,11 From clinical experience, at least 75% of dogs with GI pythiosis will go into remission with medical treatment. Treatment with mefenoxam, an agricultural fungicide, has been described.12,13 No study has shown clear benefits of including mefenoxam in addition to standard therapy (itraconazole, terbinafine, and prednisolone). Considering the risks of pet-owners handling this EPA approved fungicide, the author only recommends it’s use when standard treatment is unsuccessful. The use of a Pythium immunotherapeutic has also been described.14,15 Like mefenoxam, there is no available data suggesting immunotherapy provides any benefit over standard treatment. One study showed that vaccination did not induce measurable anti- Pythium antibodies, suggesting it will not affect the IgG assay used for diagnosis and treatment monitoring.16 This does not necessarily argue against the efficacy of immunotherapy however, as cell mediated immunity is likely even more important. The author has used immunotherapy with anecdotal success in select cases of pythiosis in dogs. More research is needed in this area. Pythium IgG levels decrease and normalize with successful treatment, along with clinical signs and abdominal imaging can be used to monitor treatment. (Table 3)
Table 1. Common Findings of pythiosis in dogs and cats.
Table 2. Medical Treatment of GI pythiosis.
Table 3. Treatment Monitoring. All of the following should be met before discontinuing treatment in dogs and cats with pythiosis.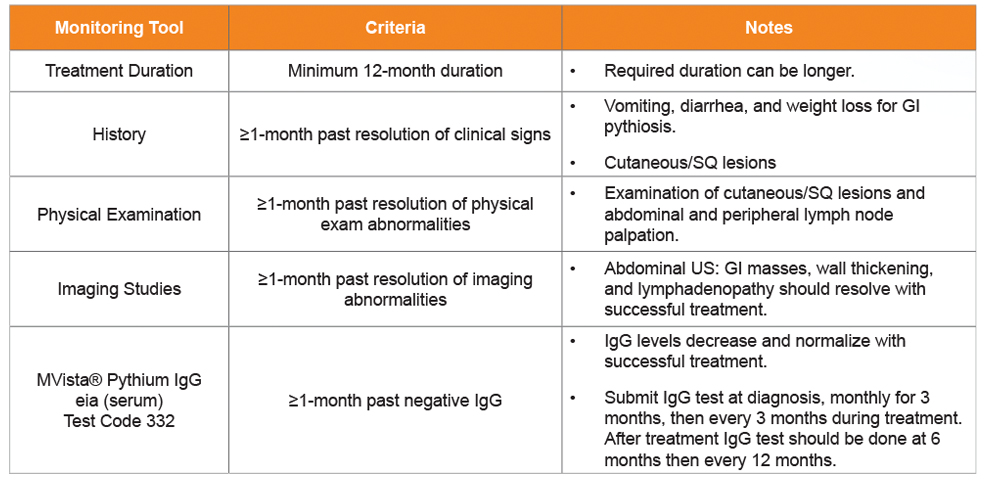
Figure 1. Geographic Location of pythiosis cases in dogs between 2000-2005 and 2016-2020 (N=469).4 The darker the color the relative higher number of cases. This image shows that pythiosis can be diagnosed in most states in the U.S., with the highest number of cases in the South.
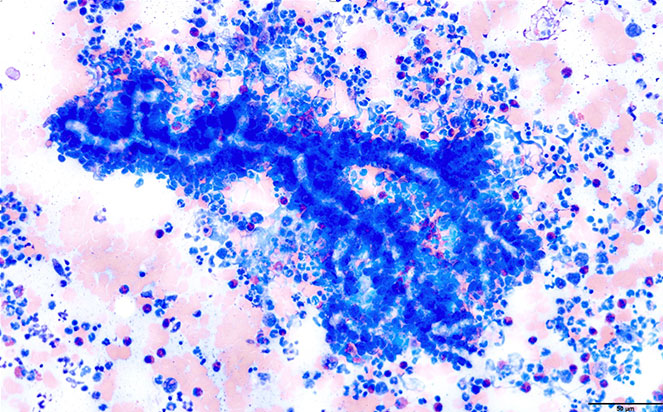
Figure 2. Photomicrograph of an aspirate smear from the perivulvar swelling pictured in Figure 5. The young-adult dog was diagnosed with pythiosis and pyogranulomatous and eosinophilic inflammation. Negatively staining hyphae, which measure ~6 μm in width and have non-parallel walls and acute- to right-angle branching, are outlined by numerous neutrophils and eosinophils (x50 objective, Wright-Giemsa). Courtesy of Dr. Shannon Dehghanpir, DACVP, LSU.
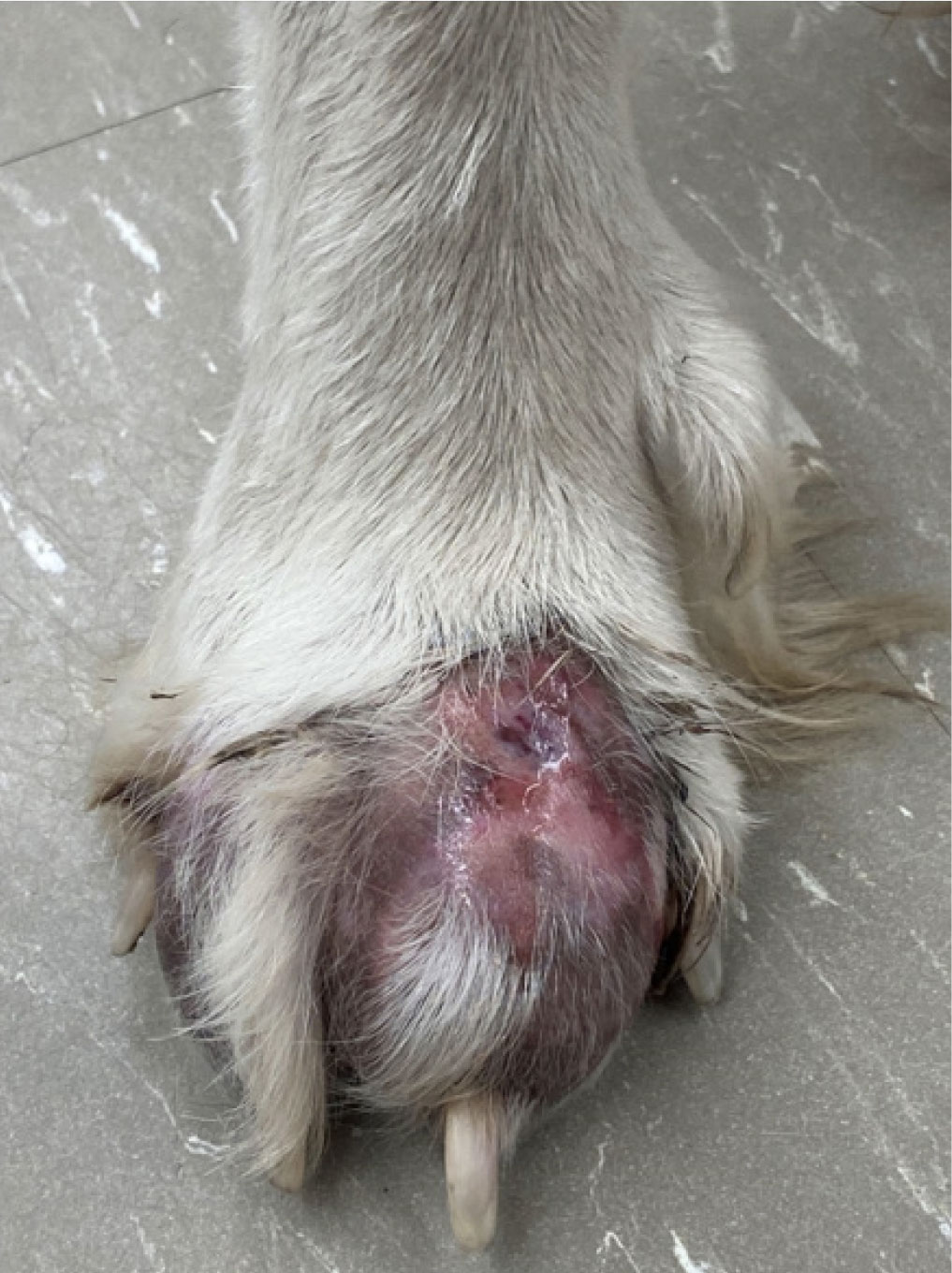
Figure 3. Ulcerative cutaneous lesion with draining tracts on
the foot of a dog with pythiosis.
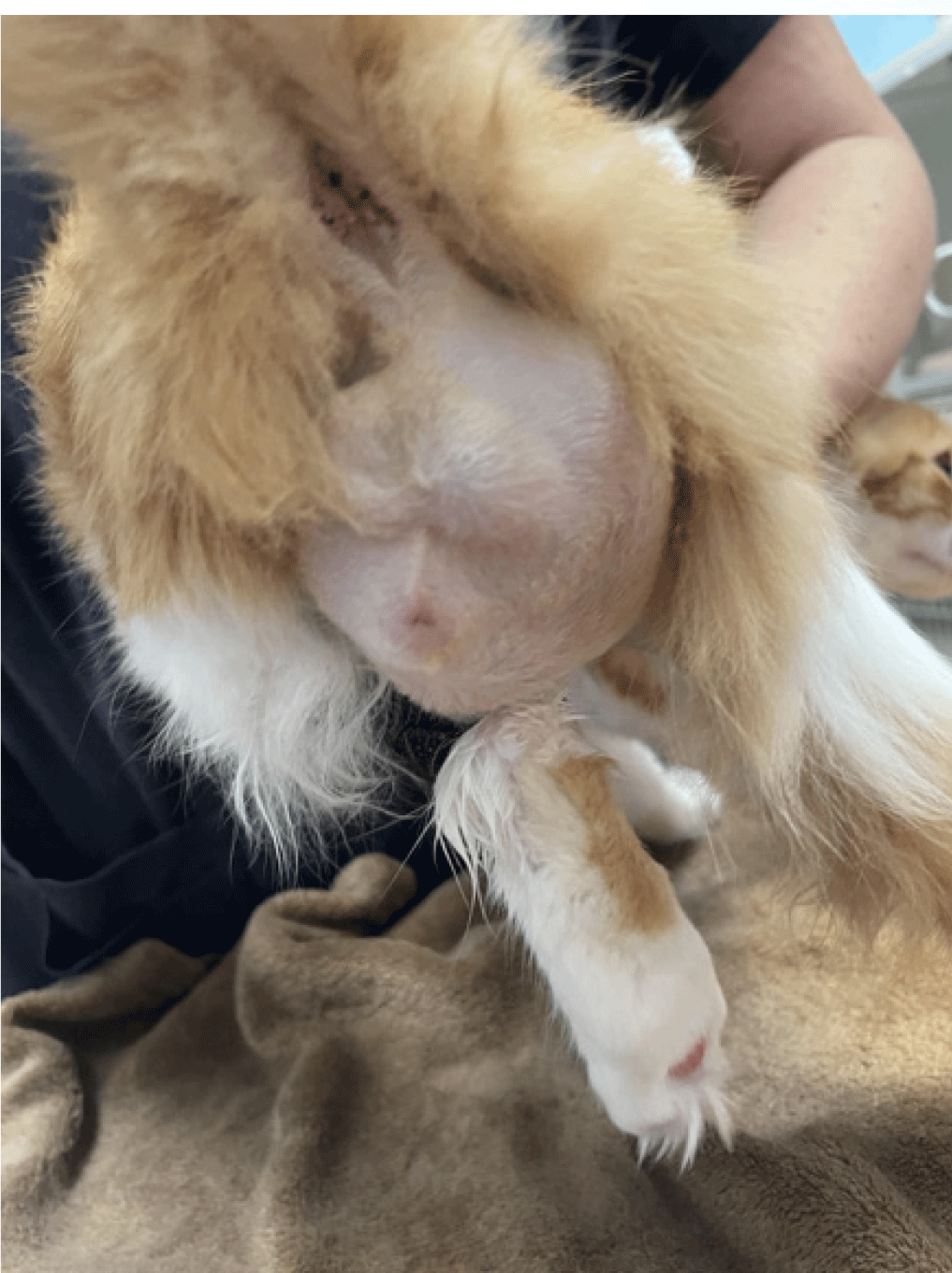
Figure 4. Large sub-cutaneous mass in the perineum in a
cat with pythiosis.
Figure 5. A. Vulvar swelling in a dog with urogenital pythiosis. B. Image shows gross hematuria due to urogenital pythiosis.
Figure 6. Abdominal ultrasound image of GI pythiosis in a dog. This image shows a large heterogenous mesenteric mass including mesenteric lymph nodes. Histoplasmosis, other infectious disease, along with cancer can cause similar lesion.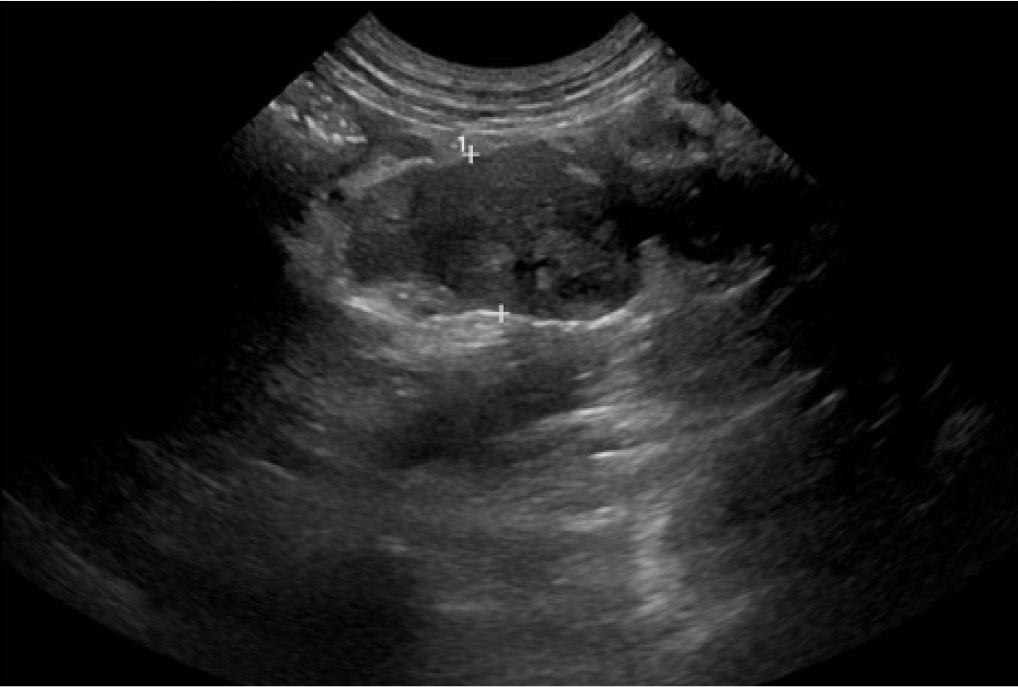
Figure 7. Abdominal ultrasound image of GI pythiosis in a dog. This image shows small intestine with a thickened wall and loss of layering suggestive of infiltrative disease. There is also free abdominal effusion. Histoplasmosis and cancer can cause similar appearing lesion.
Figure 8. Abdominal ultrasound image of a dog with GI pythiosis. This image shows a severely, diffusely thickened colon wall with a loss of layering. GI histoplasmosis and cancer can cause a similar lesion.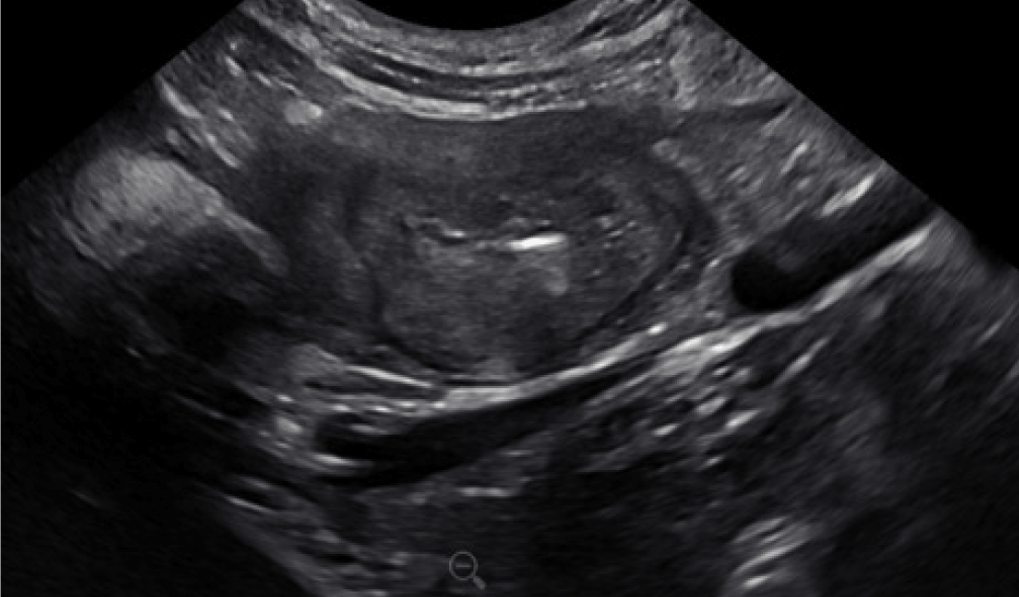
REFERENCES:
- Miraglia BM, Mendoza L, Rammohan R, et al. Pythium insidiosum complex hides a cryptic novel species: Pythium periculosum. Fungal Biol 2022;126:366-374.
- Yolanda H, Krajaejun T. Global distribution and clinical features of pythiosis in humans and animals. J Fungi (Basel) 2022;8.
- de Souto EPF, Kommers GD, Souza AP, et al. A retrospective study of pythiosis in domestic animals in northeastern Brazil. J Comp Pathol 2022;195:34-50.
- Nguyen D, Vilela R, Miraglia BM, et al. Geographic distribution of Pythium insidiosum infections in the United States. J Am Vet Med Assoc 2022;260:530-534.
- Grooters AM. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet Clin North Am Small Anim Pract 2003;33:695-720, v.
- Berryessa NA, Marks SL, Pesavento PA, et al. Gastrointestinal pythiosis in 10 dogs from California. J Vet Intern Med
2008;22:1065-1069. - Graham JP, Newell SM, Roberts GD, et al. Ultrasonographic features of canine gastrointestinal pythiosis. Vet Radiol Ultrasound 2000;41:273-277.
- Kepler D, Cole R, Lee-Fowler T, et al. Pulmonary pythiosis in a canine patient. Vet Radiol Ultrasound 2019;60:E20-E23.
- Thieman KM, Kirkby KA, Flynn-Lurie A, et al. Diagnosis and treatment of truncal cutaneous pythiosis in a dog. J Am Vet Med Assoc 2011;239:1232-1235.
- Sukanan P, Suparp B, Yongsiri S, et al. Successful management of colonic pythiosis in two dogs in Thailand using antifungal therapy. Vet Med Sci 2022;8:2283-2291.
- Reagan KL, Marks SL, Pesavento PA, et al. Successful management of 3 dogs with colonic pythiosis using itraconzaole, terbinafine, and prednisone. J Vet Intern Med 2019;33:1434-1439.
- Hummel J, Grooters A, Davidson G, et al. Successful management of gastrointestinal pythiosis in a dog using itraconazole, terbinafine, and mefenoxam. Med Mycol 2011;49:539-542.
- Cridge H, Hughes SM, Langston VC, et al. Mefenoxam, itraconazole, and terbinafine combination therapy for management of pythiosis in dogs (Six Cases). J Am Anim Hosp Assoc 2020;56:307.
- Schmiedt CW, Stratton-Phelps M, Torres BT, et al. Treatment of intestinal pythiosis in a dog with a combination of marginal excision, chemotherapy, and immunotherapy. J Am Vet Med Assoc 2012;241:358-363.
- Pereira DI, Botton SA, Azevedo MI, et al. Canine gastrointestinal pythiosis treatment by combined antifungal and immunotherapy and review of published studies. Mycopathologia 2013;176:309-315.
- Arsuaga-Zorrilla CB, Grooters AM, Pucheu-Haston CM. Quantitation of anti-Pythium insidiosum antibodies before and after administration of
an immunotherapeutic product to healthy dogs. Am J Vet Res 2018;79:1160-1165.