Itraconazole or Fluconazole – Which to Choose?
Case Example
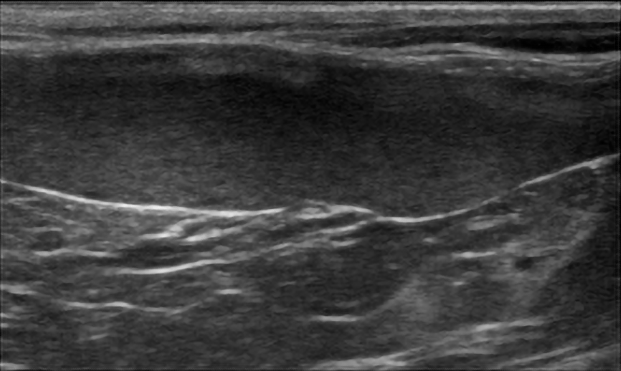
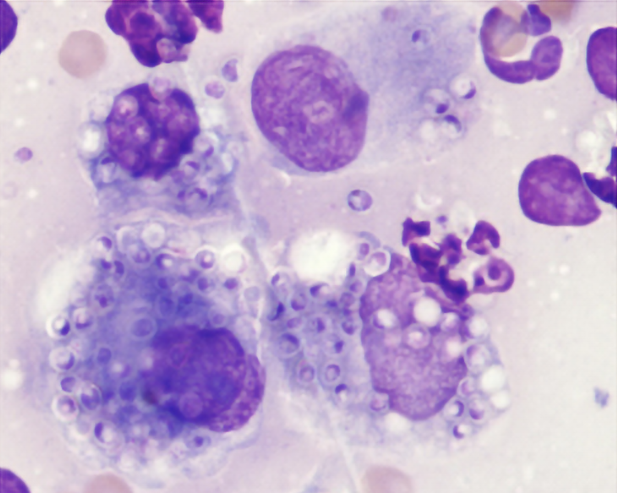
Daphne, a 6-year-old spayed, female domestic shorthair cat presented for lethargy, anorexia, weight loss, and rapid breathing. On physical examination she had generalized peripheral lymphadenopathy with the submandibular and popliteal lymph nodes being the most obviously enlarged and firm. She was tachypneic (60 bpm), had abdominal organomegaly, and was febrile 104.2F. Routine lab work showed a mild anemia (28%, RI 29-48), neutrophilia (17,910/mcL, RI 2500-8500) with a left shift (199/mcL, RI<150), and lymphopenia (1194 /mcL, RI 1200-8000). Abdominal ultrasound showed a large hypoechoic spleen with a mottled echotexture (Figure 1). Cytology of fine needle aspirate of the popliteal lymph nodes showed Histoplasma yeasts and pyogranulomatous inflammation (Figure 2).
Daphne was started on fluconazole (FDA generic, 18 mg/kg/day) and a tapering course of prednisolone. Over the first couple months she improved, but then showed signs of progressive disease as evidenced by enlarging lymph nodes and new skin lesions. Repeat cytology of fine needle aspirates of lymph node and skin impressions showed pyogranulomatous inflammation and Histoplasma yeasts. Daphne was switched to itraconazole (Itrafungol®, 5 mg/kg/day) and again started to improve. After 9 months of treatment she was considered in remission based on a resolution of clinical signs and other findings of histoplasmosis along with a negative urine MVista® Histoplasma Antigen Quantitative EIA (code 310).
Figure 1: Ultrasound image showing a large hypoechoic spleen with mottle echotexture in a cat with histoplasmosis.
Figure 2: Histoplasma yeasts seen mostly within macrophages. Wright’s stain. Photo courtesy of Jim Meinkoth.
Discussion
Daphne’s case is a good example of the 2 most common oral antifungal drugs used to treat invasive fungal infections (IFIs) – itraconazole and fluconazole. Daphne only transiently responded to fluconazole and was switched to itraconazole after approximately 3 months of therapy. Lack of response to fluconazole is expected for some dogs and cats with histoplasmosis or blastomycosis.
There are several considerations when choosing which antifungal drug to treat an IFI. Some of these include:
- Animal size – available drug formulations
- Infecting fungal organism – sensitivity to antifungal drug
- Organ systems involved – tissue penetration of antifungal drug
- Drug adverse effects – tolerability of drug treatment
- Cost of treatment – drug and treatment monitoring cost
Animal size – available drug formulations
With availability of FDA approved capsule and solution for both drugs, this is arguably less of a consideration currently. Administration of a solution can be challenging in some animals, particularly cats. This is especially pertinent as the only FDA approved itraconazole capsule size is a 100 mg, which is too large for daily administration to small dogs and cats. There are 2 potential strategies for this. The first includes opening an FDA approved capsule and spreading a portion of it over food. For example, if the desired dose is 50 mg, then ½ the capsule contents would be spread over food. Alternatively, a portion of the capsule contents can be placed into smaller capsules [1Mawby DI, Whittemore JC, Fowler LE, et al. Comparison of absorption characteristics of oral reference and compounded itraconazole formulations in healthy cats. J Am Vet Med Assoc 2018;252:195-200.]. A second option includes every other day administration of 100 mg FDA approved capsules, which has been shown to achieve therapeutic drug levels in healthy research cats [2Middleton SM, Kubier A, Dirikolu L, et al. Alternate-day dosing of itraconazole in healthy adult cats. J Vet Pharmacol Ther 2016;39:27-31.]. Similar data is not available for dogs. In both dogs and cats, monitoring itraconazole blood levels is recommended to ensure therapeutic blood levels. Compounding itraconazole from bulk chemical (even FDA approved bulk chemical for compounding) should be avoided, as GI absorption is very low [1Mawby DI, Whittemore JC, Fowler LE, et al. Comparison of absorption characteristics of oral reference and compounded itraconazole formulations in healthy cats. J Am Vet Med Assoc 2018;252:195-200.,3Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.,4Mawby DI, Whittemore JC, Genger S, et al. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med 2014;28:72-77.]. Dosing fluconazole in dogs and cats is relatively simple given the FDA approved solution and multiple tablet sizes. There is no available data on the GI absorption of compounded fluconazole.
Infecting fungal organism – sensitivity to antifungal drug
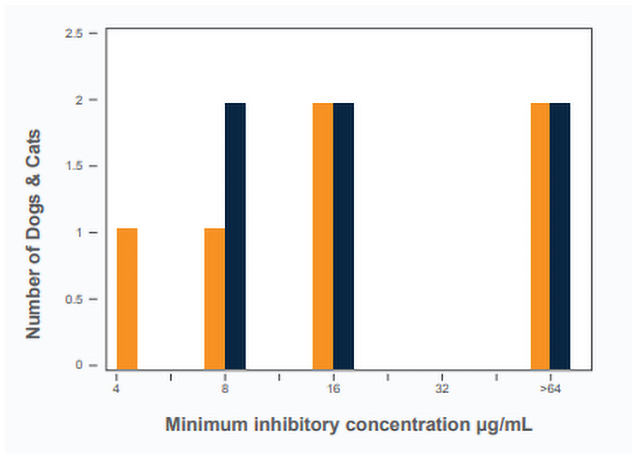
In many cases antifungal sensitivity data is not available for a particular fungal isolate. This is not a huge detriment to initial clinical decision making as the dimorphic endemic fungi (Blastomyces, Histoplasma, Coccidioides) have relatively predictable antifungal sensitivity patterns. In general, all 3 organisms are inherently much more sensitive to itraconazole as compared with fluconazole [5Gonzalez GM. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Medical mycology 2009;47:71-76.,6Yamazaki T, Inagaki Y, Fujii T, et al. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int J Antimicrob Agents 2010;36:324-331.,7Thompson GR, 3rd, Barker BM, Wiederhold NP. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob Agents Chemother 2017;61.]. It is common for the minimum inhibitory concentration (MIC) to be at least 1000-fold higher (less sensitive) for fluconazole as compared with itraconazole in the same isolate. One study showed that 8% of Coccidioides isolates had an MIC ≥32 µg/mL for fluconazole [7Thompson GR, 3rd, Barker BM, Wiederhold NP. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob Agents Chemother 2017;61.]. Based on expected blood and tissue concentrations of fluconazole (mean maximal blood concentrations = 26 µg/mL in dogs and 32 µg/mL in cats), these isolates are highly unlikely to be cleared with fluconazole therapy [8KuKanich K, KuKanich B, Lin Z, et al. Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease. J Vet Pharmacol Ther 2020.]. Similar patterns are seen for Histoplasma and Blastomyces. For example, Figure 1 shows MIC results for fluconazole in Histoplasma isolated from dogs and cats with histoplasmosis. One-third of the isolates have an MIC >64 µg/mL [8KuKanich K, KuKanich B, Lin Z, et al. Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease. J Vet Pharmacol Ther 2020.]. In addition to inherent resistance, acquired resistance to fluconazole, but not itraconazole, has been demonstrated [9Renschler JS, Norsworthy GD, Rakian RA, et al. Reduced susceptibility to fluconazole in a cat with histoplasmosis. JFMS Open Rep 2017;3:2055116917743364.].
Figure 3: Minimum inhibitory concentrations of Histoplasma isolates to fluconazole collected from dogs (orange) and cats (blue) with histoplasmosis.
Superior antifungal activity is the primary reason itraconazole is considered the first drug-of-choice for blastomycosis and histoplasmosis. A strong argument can also be made for coccidioidomycosis. If used to treat any of these IFIs, higher doses of fluconazole should be used (20 mg/kg/day) and treatment failures in some animals should be expected.
Organ systems involved – tissue penetration of antifungal drug
Essentially any organ can be affected by IFIs. There is a paucity of information regarding tissue penetration of antifungal drugs in veterinary medicine. Some educated assumptions can be made based on the small amount of published data available, extrapolation human data, and what is known about the chemistry of the antifungal drugs. Fluconazole has a wide distribution into most tissues due to its relatively small molecular size and low protein binding. This might be advantageous for immunoprotected sites such as the eye and brain. As discussed above the relative resistance of dimorphic fungi to fluconazole might negate this perceived advantage. Itraconazole is more fat-soluble, a larger molecule and more highly protein bound. Thus, it enters immunoprotected sites at lower concentrations as compared with fluconazole. Itraconazole enters some tissues at higher concentrations as compared to fluconazole including bone, joints and lung. This should be taken into consideration when treating fungal pneumonia or osteomyelitis.
Table 1. Tissue concentrations (as related to blood concentrations) of certain azole antifungal drugs [10Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014;27:68-88.,11Lemetayer JD, Dowling PM, Taylor SM, et al. Pharmacokinetics and distribution of voriconazole in body fluids of dogs after repeated oral dosing. J Vet Pharmacol Ther 2015;38:451-456.,12Vaden SL, Heit MC, Hawkins EC, et al. Fluconazole in cats: pharmacokinetics following intravenous and oral administration and penetration into cerebrospinal fluid, aqueous humour and pulmonary epithelial lining fluid. J Vet Pharmacol Ther 1997;20:181-186.]. Blue boxes indicate the drug enters the site in higher concentrations, light grey boxes indicated intermediate concentrations and dark grey boxes indicate lower concentrations. Bolded numbers are derived from studies including dogs or cats.
| Drug | Mol Wt. (g/mol) | Protein Binding (%) | Lung | Liver | Spleen | Skin | CSF | Bone | Synov |
|---|---|---|---|---|---|---|---|---|---|
| FLU | 305 | 12 | 1.2 | 3.4 | 6.1 | 11-40 | 0.9 | 0.33 | 0.88 |
| ITRA | 706 | 99.8 | 0.9-7 | 3.5 | 1.0-3.1 | .05-8 | 0.002-12 | 4.7 | 0.5-5 |
| VORI | 349 | 51 | 0.3-3.2 | 1.1-7.4 | 0.4-3.5 | NA | 0.2 | 5 | 0.22 |
| POSA | 700 | 97 | >5 | NA | NA | 0.5-5 | <0.5 | NA | NA |
Drug adverse effects – tolerability of drug treatment
Adverse effects are reported in 0-25% of dogs and cats receiving itraconazole [13Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.,14Hanzlicek AS, Meinkoth JH, Renschler JS, et al. Antigen Concentrations as an Indicator of Clinical Remission and Disease Relapse in Cats with Histoplasmosis. J Vet Intern Med 2016;30:1065-1073.,15Hodges RD, Legendre AM, Adams LG, et al. e J Vet Intern Med 1994;8:409-413.]. The most common adverse effects are GI upset (vomiting, diarrhea, anorexia), hepatotoxicity, and ulcerative cutaneous lesions [13Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.]. Hepatoxicity and skin lesions are dose dependent, more common with excessive blood levels [13Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.]. With appropriate therapeutic drug monitoring via itraconazole blood levels, many adverse effects can be avoided. This limits the need for “drug holidays” and switching to less effective drugs. Moreover, therapeutic drug monitoring helps guide individualized patient care and augments treatment cost-efficiency.
Cost of treatment – drug and treatment monitoring costs
Cost is often a major factor in diagnostic and treatment decisions in veterinary medicine. Antifungal drugs have notoriously been expensive, especially when they are new- before FDA generic options are available. Combined, the required long treatment durations and newer (innovator) antifungal drugs are often cost prohibitive. This has led some to prescribe compounded drug. In the case of itraconazole, there is a large amount of data showing this should be avoided [1Mawby DI, Whittemore JC, Fowler LE, et al. Comparison of absorption characteristics of oral reference and compounded itraconazole formulations in healthy cats. J Am Vet Med Assoc 2018;252:195-200.,3Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.,4Mawby DI, Whittemore JC, Genger S, et al. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med 2014;28:72-77.]. Less is known about the pharmacokinetics of compounded fluconazole. More recently the availability of FDA generic itraconazole (capsule and solution), in addition to drug discount programs (Ex., GoodRx), has made the cost of itraconazole similar to fluconazole. In addition, the FDA approved itraconazole solution (Itrafungol®, Elanco), labelled to treat dermatophytosis in cats, is essentially identical to the innovator product (Sporanox®), and is considerably less expensive. Table 2 below provides examples of monthly antifungal drug costs, which will vary from pharmacy-to-pharmacy. Itraconazole requires therapeutic drug monitoring to help decrease adverse effects yet ensure therapeutic levels. The cost of treatment monitoring is easily recouped by the cost-savings associated with individualized care when itraconazole blood levels are measured by bioassay (MVista® Itraconazole Bioassay), which only costs <$50/test.
Table 2. Example cost of itraconazole and fluconazole for typical doses in 20 kg dog and 5 kg cat. Prices include a discount program (GoodRx).
| Animal | 20 kg dog | 20 kg dog | 5 kg cat | 20 kg dog | 5 kg cat | 5 kg cat | 5 kg cat |
|---|---|---|---|---|---|---|---|
| Medication | Itra | Itra | Itra | Itra | Itra | Flu | Flu |
| Type | Sporanox® | Generic | Sporanox® | ItraFungol® | Generic | Generic | Generic |
| Form | Capsule | Capsule | Solution | Solution | Solution | Tablet | Solution |
| Strength | 100 mg | 100 mg | 10 mg/ml | 10 mg/ml | 10 mg/ml | 200 mg | 40 mg/ml |
| mg/kg/day | 5 | 5 | 5 | 5 | 5 | 20 | 20 |
| $ Costco | $561 | $39 | $173 | $89 | $67 | $45 | |
| $ CVS | $594 | $90 | $184 | $51 | $142 | $78 | |
| $ Walmart | $593 | $53 | $183 | $48 | $82 | $61 | |
| $ Chewy | $76 | ||||||
| Lowest Cost | $561 | $39 | $173 | $76 | $48 | $67 | $45 |
Table 3. Fluconazole and itraconazole dosing in dogs and cats.
| Disease | Severity | Treatment | Initial Dose |
|---|---|---|---|
| Histoplasmosis Blastomycosis |
Severe Life-threatening |
Lipid or liposomal amphotericin B^ |
0.5 −1.0 mg/kg IV EOD (cat) 1.0-1.5 mg/kg IV EOD (dog) up to cumulative 12 mg/kg (cat) and 24 mg/kg (dog) |
| Mild-Moderate (First Choice) |
Itraconazole*# | Cap: 10 mg/kg/day Cap: 5 mg/kg/day Sol: 5 mg/kg/day (cat) |
|
| Mild-Moderate (Second Choice) |
Fluconazole# | 20 mg/kg/day | |
| Cryptococcosis Coccidioidomycosis$ |
Severe Life-threatening |
Lipid or liposomal amphotericin B^ |
0.5-1.0 mg/kg IV EOD (cat) 1.0 – 1.5 mg/kg IV EOD (dog) up to cumulative 12 mg/kg (cat) and 24 mg/kg (dog) |
| Mild-Moderate (First Choice) |
Fluconazole# | 20 mg/kg/day PO | |
| Mild-Moderate (Second Choice) |
Itraconazole*# | Cap: 10 mg/kg/day (cat) Cap: 5 mg/kg/day (dog) Sol: 5 mg/kg/day (cat) |
Cap, capsule; Sol, solution; EOD, every other day
$ Some consider itraconazole the first-choice antifungal for coccidioidomycosis.
^ Adequate hydration should be maintained and kidney values should be checked before each dose. Start at low end of dose and if well tolerated dose can be increased.
* This is a starting dose only. Dose should be individualized based on itraconazole blood levels after steady state (3 weeks after starting drug).
# Monitor liver enzymes during treatment.
REFERENCES:
- Mawby DI, Whittemore JC, Fowler LE, et al. Comparison of absorption characteristics of oral reference and compounded itraconazole formulations in healthy cats. J Am Vet Med Assoc 2018;252:195-200.
- Middleton SM, Kubier A, Dirikolu L, et al. Alternate-day dosing of itraconazole in healthy adult cats. J Vet Pharmacol Ther 2016;39:27-31.
- Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.
- Mawby DI, Whittemore JC, Genger S, et al. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med 2014;28:72-77.
- Gonzalez GM. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Medical mycology 2009;47:71-76.
- Yamazaki T, Inagaki Y, Fujii T, et al. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int J Antimicrob Agents 2010;36:324-331.
- Thompson GR, 3rd, Barker BM, Wiederhold NP. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob Agents Chemother 2017;61.
- KuKanich K, KuKanich B, Lin Z, et al. Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease. J Vet Pharmacol Ther 2020.
- Renschler JS, Norsworthy GD, Rakian RA, et al. Reduced susceptibility to fluconazole in a cat with histoplasmosis. JFMS Open Rep 2017;3:2055116917743364.
- Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014;27:68-88.
- Lemetayer JD, Dowling PM, Taylor SM, et al. Pharmacokinetics and distribution of voriconazole in body fluids of dogs after repeated oral dosing. J Vet Pharmacol Ther 2015;38:451-456.
- Vaden SL, Heit MC, Hawkins EC, et al. Fluconazole in cats: pharmacokinetics following intravenous and oral administration and penetration into cerebrospinal fluid, aqueous humour and pulmonary epithelial lining fluid. J Vet Pharmacol Ther 1997;20:181-186.
- Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.
- Hanzlicek AS, Meinkoth JH, Renschler JS, et al. Antigen Concentrations as an Indicator of Clinical Remission and Disease Relapse in Cats with Histoplasmosis. J Vet Intern Med 2016;30:1065-1073.
- Hodges RD, Legendre AM, Adams LG, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med 1994;8:409-413.