Invasive Fungal Infections
NOT Invasive Diagnostic Investigations
(1,3)-β-D-Glucan Antigen Test in Dogs and Cats
Andrew Hanzlicek, DVM, MS, DACVIM (SAIM)
Clinical Challenge
Invasive fungal infections (IFI) often present a diagnostic challenge in dogs and cats. A few of the most notable include variable clinical presentations without pathognomonic clinical findings; disease in organ systems that require invasive procedures for sample collection; and slow fungal culture results that lack sensitivity.
More recently non-invasive tests to help support the diagnosis of an IFI have been developed. Some examples include the urine antigen enzyme immunoassay (EIA) for histoplasmosis or blastomycosis, the serum antigen latex agglutination test for cryptococcosis, and the serum antibody EIA or urine antigen EIA for coccidioidomycosis. Most of these tests are relatively specific to the fungal organism of interest. As such, there are countless fungal organisms that can cause an IFI for which specific non-invasive tests do not exist.
Non-Invasive Testing Option
For many of the aforementioned organisms, the serum β-Dglucan (BDG) antigen test provides a non-invasive diagnostic option. Think of it as a pan-fungal antigen test, including infections with molds, yeasts, and fungal-like organisms such as oomycetes and Pneumocystis spp [1
Data in Dogs and Cats
The clinical performance of serum BDG was investigated in healthy dogs and cats and animals with histoplasmosis, coccidioidomycosis, systemic aspergillosis, and other systemic molds [8
Table 1. Diagnostic performance of serum β-D-Glucan antigen test in dogs and cats with various confirmed IFIs, with other (non-fungal) disease, and healthy controls [8
| Diagnosis | Total Animals (N =256) |
Overall Sensitivity (%) |
Overall Specificity (%) |
Antigen Specificity (%) |
|---|---|---|---|---|
| Histoplasmosis | 27a | 88 | 100 (urine)* | |
| Coccidioidomycosis | 27b | 44 | 48* | |
| Aspergillosis | 27c | 77 | 77* | |
| Mold (other) | 4d | 100 | ||
| Non-fungal disease | 112e | 72 | ||
| Healthy controls | 59f | 88 |
*Urine antigen testing= MiraVista Histoplasma urine antigen enzyme immunoassay; Antigen testing= MiraVista Coccidioides antigen enzyme immunoassay; Antigen testing= Platelia, antigen enzyme immunoassay. Dogs (Cats) in study: a Histoplasmosis 7(20), b Coccidioidomycosis 23(4), c Aspergillosis 27 dogs, d Mold (other) 4 dogs, e Non-fungal disease 99(13), f Healthy controls 55(4)
Indications For β-D-Glucan Antigen Test
While there remains a paucity of published data in veterinary medicine, based on data in other species, serum BDG antigen testing should be considered in the following clinical scenarios.
- Where IFI is suspected and a definitive diagnosis cannot be obtained via cytopathology, histopathology or fungal culture [1
He S, Hang JP, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-D-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect 2015;48:351-361. ]. - Where IFI is suspected and testing for enzootic dimorphic fungi (Histoplasma, Blastomyces, Cryptococcus, Coccidioides) in the geographic area are negative [1
He S, Hang JP, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-D-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect 2015;48:351-361. ]. - Where invasive aspergillosis is suspected and the Aspergillus antigen EIA is negative [9
Lahmer T, Neuenhahn M, Held J, et al. Comparison of 1,3-beta-d-glucan with galactomannan in serum and bronchoalveolar fluid for the detection of Aspergillus species in immunosuppressed mechanical ventilated critically ill patients. J Crit Care 2016;36:259-264. , 10Sarwar M, Gardezi SAH, Zaman G, et al. Evaluation of galactomannan and beta-d-glucan assays for the diagnosis of invasive aspergillosis in clinically suspected cases. J Pak Med Assoc 2020;70:442-446. ]. - For treatment monitoring of IFI when BDG antigen test is positive at baseline [2
Permpalung N, Worasilchai N, Chindamporn A. Human pythiosis: Emergence of fungal-like organism. Mycopathologia 2019. , 4Nucci M, Barreiros G, Reis H, et al. Performance of 1,3-beta-D-glucan in the diagnosis and monitoring of invasive fusariosis. Mycoses 2019;62:570-575. , 11Ito-Takeichi S, Niwa T, Fujibayashi A, et al. The impact of implementing an antifungal stewardship with monitoring of 1-3, beta-D-glucan values on antifungal consumption and clinical outcomes. J Clin Pharm Ther 2019;44:454-462. ]. - For treatment monitoring of pneumocystosis when BDG antigen test is positive at baseline [12
McCarthy MW, Petraitiene R, Walsh TJ. Translational Development and Application of (1–>3)-beta-D-Glucan for diagnosis and therapeutic monitoring of Invasive mycoses. Int J Mol Sci 2017;18. ].
Case Presentation
Lady is an 11-year-old spayed, female cocker spaniel that was presented for tremoring and acting painful as evidenced by walking with a hunched posture. Physical examination revealed pain on lateral extension of the neck to the right and paraspinal palpation in the caudal thoracic region. The remainder of the physical examination was unremarkable. Routine lab work showed moderate hyperglobulinemia, mild hypoalbuminemia, moderate mature neutrophilia and monocytosis, collectively suggesting chronic inflammation.
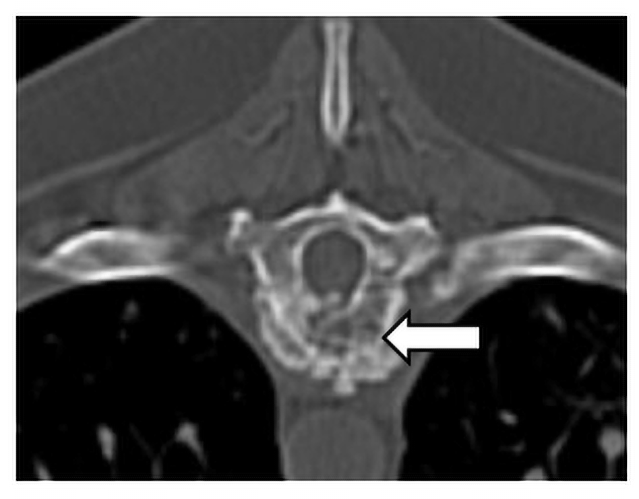
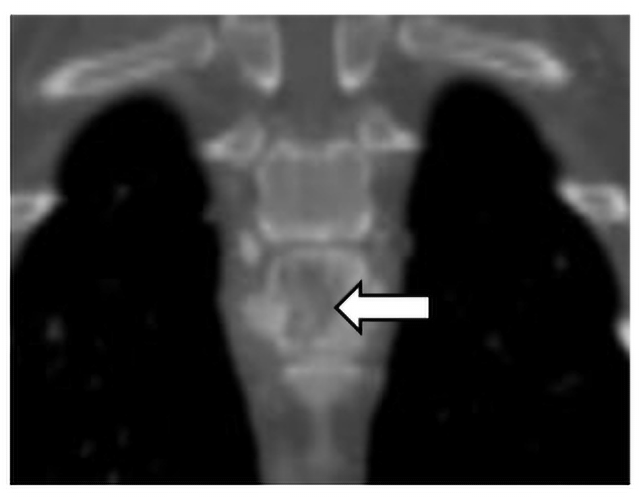
Cervical and thoracic CT showed multiple lytic and proliferative bone lesions in the cervical and thoracic vertebrae (Figures 1 and 2) and a rib. Neoplasia or IFI was suspected. CT guided aspirate of the lesions showed mixed inflammation, but no evidence of neoplasia and no fungal organisms. The pet-owner was unable to pursue surgical biopsies. Due to suspicion of IFI, serum BDG test was submitted to MiraVista Diagnostics and was positive (125 pg/mL; normal< 60 pg/mL). Antifungal therapy, in addition to multi-modal treatment for pain, was instituted and clinical signs improved, but never completely resolved. Approximately 10 months later, Lady died of an unrelated cause. In multiple vertebrae, necropsy and histopathology revealed granulomatous inflammation with aggregates of macrophages containing large yeasts. Fungal culture and/or molecular diagnostics were not performed to speciate the fungal organism.
In Lady’s case a positive serum BDG test was supportive of the clinical suspicion of IFI. Since more invasive testing was not feasible, appropriate treatment was instituted. Monitoring serum BDG concentrations in addition to clinical signs and potentially repeat imaging could be useful for guiding treatment.
Figure 1. Transverse plane CT image of the osteolytic and proliferative lesion (white arrow) in the ninth thoracic vertebrae caused by an invasive IFI.
Figure 2. Dorsal plane CT image of the osteolytic and proliferative lesion (white arrow) in the ninth thoracic vertebrae caused by an IFI.
(1,3)-β-D-Glucan Antigen Test at MiraVista Diagnostics
Test Code
317
Clinical Significance
The Fungitell® assay measures the presence of (1→3)-β-D-Glucan in serum and CSF. Serum is the only specimen type cleared by the FDA for this assay. Performance characteristics of CSF have been validated by MiraVista Diagnostics.
Methodology
Colorimetric Fungitell® assay utilizing a (1→3)-β-D-Glucan-specific Limulus Amebocyte Lysate (LAL) reagent.
Specimen Collection
Collect serum specimens in serum separator or red top tube. Allow blood to clot for 30 minutes, then centrifuge. Pipette serum into a transport tube without interfering levels of (1→3)-β-D-Glucan. Most sterile polypropylene DNAase and RNAse free tubes are acceptable.
Minimum Specimen Requirements
Serum: 0.2 mL
Specimen Stability
Room Temperature: Not acceptable
Refrigerated: 5 days
Frozen: Indefinitely
Specimen Rejection
Lipemic, hemolyzed or icteric specimens may be rejected due to interference
Transport Temperature
Refrigerated/Frozen
Shipping
Ship on dry ice or frozen packs for next day service. Monday – Friday delivery
Turnaround Time
Testing is performed Monday – Friday
Serum: 1-2 days
REFERENCES:
- He S, Hang JP, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-D-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect 2015;48:351-361.
- Permpalung N, Worasilchai N, Chindamporn A. Human pythiosis: Emergence of fungal-like organism. Mycopathologia 2019.
- Petraitiene R, Petraitis V, Maung BW, et al. Posaconazole alone and in combination with caspofungin for treatment of experimental exserohilum rostratum meningoencephalitis: Developing new strategies for treatment of phaeohyphomycosis of the central nervous system. J Fungi (Basel) 2020;6.
- Nucci M, Barreiros G, Reis H, et al. Performance of 1,3-beta-D-glucan in the diagnosis and monitoring of invasive fusariosis. Mycoses 2019;62:570-575.
- Morjaria S, Frame J, Franco-Garcia A, et al. Clinical performance of (1,3) beta-D-glucan for the diagnosis of pneumocystis pneumonia (PCP) in cancer patients tested with PCP polymerase chain reaction. Clin Infect Dis 2019;69:1303-1309.
- Rhein J, Bahr NC, Morawski BM, et al. Detection of High Cerebrospinal Fluid Levels of (1–>3)-beta-d-Glucan in Cryptococcal Meningitis. Open Forum Infect Dis 2014;1:ofu105.
- Weissenbacher-Lang C, Fuchs-Baumgartinger A, Guija-DeArespacochaga A, et al. Pneumocystosis in dogs: meta-analysis of 43 published cases including clinical signs, diagnostic procedures, and treatment. J Vet Diagn Invest 2018;30:26-35.
- Renschler J, Wheat L. Evaluation of (1,3)-B-D-Glucan as a biomarker of systemic fungal infections in dogs and cats. Poster presented at: American College of Veterinary Pathologists Annual Meeting. Minneapolis, MN: 2015.
- Lahmer T, Neuenhahn M, Held J, et al. Comparison of 1,3-beta-d-glucan with galactomannan in serum and bronchoalveolar fluid for the detection of Aspergillus species in immunosuppressed mechanical ventilated critically ill patients. J Crit Care 2016;36:259-264.
- Sarwar M, Gardezi SAH, Zaman G, et al. Evaluation of galactomannan and beta-d-glucan assays for the diagnosis of invasive aspergillosis in clinically suspected cases. J Pak Med Assoc 2020;70:442-446.
- Ito-Takeichi S, Niwa T, Fujibayashi A, et al. The impact of implementing an antifungal stewardship with monitoring of 1-3, beta-D-glucan values on antifungal consumption and clinical outcomes. J Clin Pharm Ther 2019;44:454-462.
- McCarthy MW, Petraitiene R, Walsh TJ. Translational Development and Application of (1–>3)-beta-D-Glucan for diagnosis and therapeutic monitoring of Invasive mycoses. Int J Mol Sci 2017;18.