Blastomycosis In Veterinary Medicine 2020
Introduction
Blastomycosis is acquired by inhaling fungal spores and causes a primarily respiratory or disseminated infection. If the inoculum is small, and the animal is immunocompetent, the infection may be limited to the respiratory tract and may cause few or no clinical signs. Alternatively, it can be fatal if not diagnosed early. Many cases may be diagnosed by identification of the yeast in cytologic or histologic samples. Early diagnosis may also be aided by detection of fungal antigen in urine or serum. This article will review the clinical features of blastomycosis in dogs and cats and discuss the current diagnostic and treatment recommendations.
Epidemiology
While blastomycosis may occur in a wide variety of animals, most diagnosed cases are in dogs. Enzootic areas for blastomycosis include the Mississippi, Ohio, and Missouri river valleys, the Eastern Seaboard, Southern Canada, and areas adjacent to the Great Lakes (Figure). The states with highest incidence are Wisconsin, Minnesota, Missouri, Illinois, Michigan, Kentucky, West Virginia, Arkansas, Tennessee, North Carolina, South Carolina, Louisiana, and Mississippi. Other endemic states include Indiana, Iowa, Ohio, Virginia, Georgia, Alabama and Vermont.
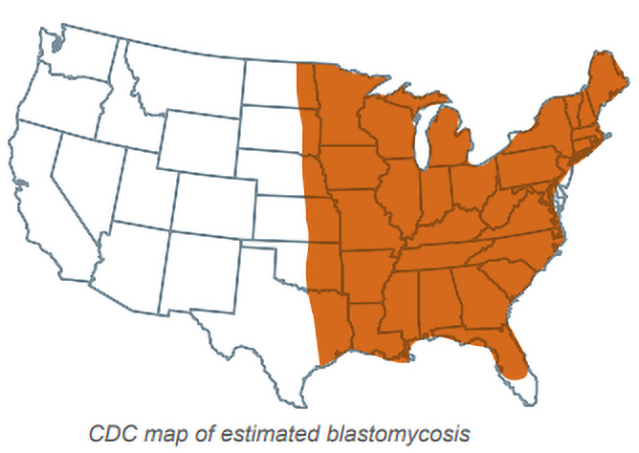
Cases, however, may occur outside the enzootic area [1Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 2005;20:227-232.]. The annual incidence was 1420 cases per 100,000 dogs in a highly enzootic area [2Baumgardner DJ, Paretsky DP, Yopp AC. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Med Vet Mycol 1995;33:171-176.]. Proximity to waterways and exposure to excavation are significant risk factors but age, sex, and activities such as hunting, swimming and exposure to beavers are not. While most cases occur in dogs with extensive outdoor exposure, cases also may be seen in indoor pets [1Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 2005;20:227-232.]. Cases occur most often in the fall but may occur any time of the year [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.,4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.].
Blastomycosis occurs mainly in young, large-breed dogs with the highest rates in Coonhounds, Pointers and Weimaraners [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.,4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.]. Doberman Pinschers and Retrievers also may be at increased risk for blastomycosis, but any breed is susceptible if exposed to the organism. In some reports, the prevalence was higher in males than females.[4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.,5Selby LA, Becker SV, Hayes HW, Jr. Epidemiologic risk factors associated with canine systemic mycoses. Am J Epidemiol 1981;113:133-139.]. Higher rates in sexually intact male dogs was thought to be caused by roaming behavior or selective use in hunting [4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.]. The incidence of feline blastomycosis is much lower than that in dogs, with one retrospective study over 11 years at a Canadian veterinary school showing 151 total blastomycosis cases, of which only 6 were feline (4%) [6Davies JL, Epp T, Burgess HJ. Prevalence and geographic distribution of canine and feline blastomycosis in the Canadian prairies. Can Vet J 2013;54:753-760.]. A 5-year survey of the Veterinary Medical Data Program found only 3 infected cats compared to 324 dogs with blastomycosis [7Legendre AM. Blastomycosis. in Infectious Diseases of the Dog and Cat. 4th ed. Greene CE Ed. St. Louis, MO: Elsevier; 2012.].
Clinical Findings
The most common clinical findings are nonspecific and include loss of appetite, weight loss, and fever (Table 1). Respiratory abnormalities also are common, and radiographs show nodular or interstitial infiltrates, often referred to as a “snowstorm pattern” [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.]. Less frequently thoracic radiographs that show tracheobronchial lymphadenopathy, masses, or cavitary lesions [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.]. Draining skin tracts and lymphadenopathy are commonly present. Among fatal cases, the organs most often involved are the lungs, eyes and skin [4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.]. Ocular lesions occur in about one third of cases [8Bloom JD, Hamor RE, Gerding PA, Jr. Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985-1993). J Am Vet Med Assoc 1996;209:1271-1274.]. Other less common sites of dissemination include the central nervous system and genitourinary tract [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.]
Early detection of the ocular lesions is important for saving vision and for diagnosing the systemic nature of the disease. In a review of cases with ocular involvement, endophthalmitis was most common, followed by posterior segment disease, and anterior segment disease [8Bloom JD, Hamor RE, Gerding PA, Jr. Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985-1993). J Am Vet Med Assoc 1996;209:1271-1274.]. Lens rupture is a potential complication [9Hendrix DV, Rohrbach BW, Bochsler PN, et al. Comparison of histologic lesions of endophthalmitis induced by Blastomyces dermatitidis in untreated and treated dogs: 36 cases (1986-2001). J Am Vet Med Assoc 2004;224:1317-1322.]. In an earlier report, the most common ocular lesion was uveitis, and other manifestations including retinal detachment, panophthalmitis, and glaucoma. The most common ocular exam findings include photophobia, conjunctival hyperemia, miosis, blepharospasm, and aqueous flare. Most of the dogs also exhibited pneumonia and many had skin lesions or enlarged lymph nodes. The presence of ocular disease in dogs from areas enzootic for blastomycosis should prompt careful evaluation for the condition.
Large surveys are not available to define the most common clinical manifestations in cats but are likely similar to those in dogs. Respiratory signs and dermal lesions were the most common findings in a study of 8 cases [10Gilor C, Graves TK, Barger AM, et al. Clinical aspects of natural infection with Blastomyces dermatitidis in cats: 8 cases (1991-2005). J Am Vet Med Assoc 2006;229:96-99.]. Chorioretinitis and CNS signs have also been reported in cats with disseminated blastomycosis [11Breider MA, Walker TL, Legendre AM, et al. Blastomycosis in cats: five cases (1979-1986). J Am Vet Med Assoc 1988;193:570-572.]. Affected cats have usually had negative retroviral status, and immunosuppression is not typically associated with development of clinical blastomycosis [10Gilor C, Graves TK, Barger AM, et al. Clinical aspects of natural infection with Blastomyces dermatitidis in cats: 8 cases (1991-2005). J Am Vet Med Assoc 2006;229:96-99.,12Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc 1996;32:380-391.].
Diagnosis.95% of cases are based on compatible clinical findings confirmed by positive laboratory tests [4Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.]. Most are based on antigen tests or pathology. The diagnostic performance of the antigen and antibody tests offered at MiraVista Diagnostics are depicted in Table 2 and recommendations for what test to order in Table 3.
Pathology and culture.Cytology and/or histopathology is a common method for diagnosis. Blastomyces organisms appear in cytologic preparations stained with Romanowskytype stains as 8-20 µm, blue, spherical, thick-walled yeasts. Broad-based budding is commonly observed. Cytology was positive in 71% of cases in one report, including mostly skin and lymph node samples, and occasionally transtracheal washes [3Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.]. In some cases, cytology of subretinal aspirates has been positive [13Buyukmihci N. Ocular lesions of blastomycosis in the dog. J Am Vet Med Assoc 1982;180:426-431.]. Fungal culture should also be performed on specimens submitted for pathology. Although data are unavailable for veterinary patients, culture often is positive while pathology is negative in humans.
Antigen detection. Blastomyces surface antigens can be detected by an enzyme immunoassay (EIA) in body fluids including urine, serum, bronchoalveolar lavage fluid, cerebrospinal fluid, etc [14Durkin M, Witt J, Lemonte A, et al. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol 2004;42:4873-4875.]. In 2006, Spector et al. demonstrated that the antigen test is also useful in dogs, showing 94% sensitivity for urine and 87% sensitivity for serum in dogs with pathology-proven blastomycosis (Table 2-SN SP) [15Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 2008;22:839-843.]. Antigen concentration correlates with the severity of the infection, and results are almost always positive in moderate-severe or severe blastomycosis. Antigen tests may initially be negative in mild or localized cases and those presenting with peracute or acute disease (1-2 weeks); therefore, a negative result does not exclude the diagnosis. In addition, nearly complete cross-reactivity occurs between antigen detection for histoplasmosis and blastomycosis, and current antigen tests cannot differentiate the two systemic fungal infections: There is no need to order testing for both antigens.
Antibody detection. Tests for antibody to Blastomyces using immunodiffusion (ID) methods have limited clinical utility. Enzyme immunoassay (EIA) methods of IgG antibody detection reportedly have higher sensitivity compared to ID, 95% and 65%, respectively [16Mourning AC, Patterson EE, Kirsch EJ, et al. Evaluation of an enzyme immunoassay for antibodies to a recombinant Blastomyces adhesin-1 repeat antigen as an aid in the diagnosis of blastomycosis in dogs. J Am Vet Med Assoc 2015;247:1133-1138.] (Table 2). Blastomyces canine IgG is available at MiraVista Diagnostics (MVista® Blastomyces Canine IgG antibody EIA). The specificity of the IgG antibody EIA is high (95% in healthy control dogs from an enzootic area, 100% in dogs with nonfungal pulmonary disease), although some cross-reactivity is observed in dogs with histoplasmosis [16Mourning AC, Patterson EE, Kirsch EJ, et al. Evaluation of an enzyme immunoassay for antibodies to a recombinant Blastomyces adhesin-1 repeat antigen as an aid in the diagnosis of blastomycosis in dogs. J Am Vet Med Assoc 2015;247:1133-1138.]. Antibody testing may be useful for diagnosis of cases with suspected false negative antigen results, or in cases with weekly positive antigen results in which the clinical findings are equivocal. There is no commercially available feline antibody EIA, but the Blastomyces ID can be used in cats.
Molecular techniques. Polymerase chain reaction (PCR) assays were demonstrated to detect Blastomyces DNA in 8/13 paraffin-embedded canine tissue samples in which yeast cells were seen microscopically, as well as in soil samples from a dog kennel housing dogs with blastomycosis [17Bialek R, Cirera AC, Herrmann T, et al. Nested PCR assays for detection of Blastomyces dermatitidis DNA in paraffin-embedded canine tissue. J Clin Microbiol 2003;41:205-208.,18Burgess JW, Schwan WR, Volk TJ. PCR-based detection of DNA from the human pathogen Blastomyces dermatitidis from natural soil samples. Med Mycol 2006;44:741-748.]. Real-time PCR assays for the B. dermatitidis BAD1 gene promotor are available from several diagnostic laboratories, and human studies have shown that Blastomyces can be detected by RT-PCR in culture specimens, paraffin-embedded tissue specimens and clinical samples (respiratory specimens, blood, bone marrow, peritoneal fluid, etc.) [19Babady NE, Buckwalter SP, Hall L, et al. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011;49:3204-3208.,20Sidamonidze K, Peck MK, Perez M, et al. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J Clin Microbiol 2012;50:1783-1786.]. The sensitivity for RT-PCR was 86% (10/12 clinical specimens) and 83% (5/6 tissue specimens) in two human studies; therefore, the number of samples was too low to make any conclusions regarding the clinical utility of RT-PCR [19Babady NE, Buckwalter SP, Hall L, et al. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011;49:3204-3208.,20Sidamonidze K, Peck MK, Perez M, et al. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J Clin Microbiol 2012;50:1783-1786.]. Additionally, no peer-reviewed publications are available to assess the utility of molecular testing for the diagnosis of canine or feline blastomycosis. The acquisition of respiratory or tissue specimens may be challenging or invasive in some cases; therefore, testing urine or serum for the presence of antigen or antibodies is often advantageous. Due to the low incidence of fungemia, whole blood is unlikely to be a desirable specimen for RT-PCR testing. When more invasive procedures are performed to obtain respiratory or tissue specimens, a diagnosis may often be obtained by cytology or histopathology.
Treatment
Amphotericin B. In severe, life-threatening cases, lipid or liposomal encapsulated (Abelcet® or Ambisome®) amphotericin B is recommended at a starting dosage of 1.0 mg/kg (dog) or 0.5 mg/kg (cat), three times weekly (or every other day) by intravenous infusion, over 4-6 hours, up to a cumulative dose of 24 mg/kg (dog) or 12 mg/kg (cat) (Table 4) [21Krawiec DR, McKiernan BC, Twardock AR, et al. Use of an amphotericin B lipid complex for treatment of blastomycosis in dogs. J Am Vet Med Assoc 1996;209:2073-2075.]. Higher doses (2-3 mg/kg/dose) might be tolerated, especially in dogs. Renal function, electrolytes, and potassium levels should be monitored during treatment. Respiratory failure is the cause of death in most cases and supplemental oxygen is appropriate. Reasons that amphotericin B is more effective than itraconazole include immediate achievement of therapeutic blood levels and its fungicidal mode of action: Itraconazole requires up to a week to reach therapeutic levels and is fungistatic. Antiinflammatory treatment with low doses of corticosteroids may be helpful in reducing systemic side effects of amphotericin B and clinical worsening attributed to inflammatory reaction to antigens released from “dying” Blastomyces organisms.
Itraconazole. In non-life-threatening cases FDA approved itraconazole is recommended [22Sykes J. Canine and Feline Infectious Disease. St. Louis, MO: Elsevier Saunders; 2019.]. Generic FDA approved itraconazole capsules are less expensive than Sporanox capsules and achieves similar concentrations in the blood.23 FDA approved itraconazole capsules should be administered with food to achieve maximum blood levels. Compounded itraconazole powder should not be used, as blood levels are undetectable or very low [22Sykes J. Canine and Feline Infectious Disease. St. Louis, MO: Elsevier Saunders; 2019.,23Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.].
The dosage to achieve therapeutic blood concentration is 5 mg/kg/d in dogs and 10 mg/kg/d in cats (7.5 mg/kg/d solution in cats), although therapeutic drug monitoring is highly recommended to attain the optimum dosage. A loading dose of 5 mg/kg q12h or 10mg/kg q24h is recommended for 3 days to achieve steady state drug concentrations more rapidly in dogs [7Legendre AM. Blastomycosis. in Infectious Diseases of the Dog and Cat. 4th ed. Greene CE Ed. St. Louis, MO: Elsevier; 2012.,22Sykes J. Canine and Feline Infectious Disease. St. Louis, MO: Elsevier Saunders; 2019.].
Itraconazole is eliminated by hepatic metabolism through cytochrome P450 3A4, and blood levels may be affected by medications that interact with that enzyme [24Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009;53:24-34.]. Itraconazole blood level measurement is recommended at steady state for all patients (day 14 of treatment for dogs and day 21 for cats) and any time treatment failure or drug toxicity is suspected.
Itraconazole blood level testing is performed by bioassay at MiraVista Diagnostics. The bioassay determines itraconazole concentration by inhibition of growth of Candida in a well of an agar plate containing the patient serum. The bioassay is simple and inexpensive. Blood levels between 2-7 µg/ ml are recommended to maximize efficacy and minimize toxicity. The primary limitation of the bioassay is that other antifungal agents will inhibit growth of a Candida and overestimate itraconazole concentration. Even when only itraconazole is administered, bioassay concentrations are higher as compared with HPLC, in part because the bioassay measures antifungal activity of itraconazole and all active metabolites (hydroxy-itraconazole, etc.).
Blood level testing using high performance liquid chromatography (HPLC) or mass spectroscopy (MS) are offered at several commercial laboratories. Trough blood levels of at least 1-2 µg/ml are recommended but specific recommendations are unavailable for the upper range [24Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009;53:24-34.]. Combined itraconazole and hydroxy-itraconazole concentrations above 5 µg/ml may be unnecessary and are more likely cause toxicity (personal communications). Some laboratories do not report the hydroxy-itraconazole metabolite and it is important to have both concentrations to assess risk for toxicity.
Itraconazole may cause a variety of adverse effects, most commonly loss of appetite, anorexia, vomiting, or diarrhea, which may be related to high blood levels [25Lestner JM, Roberts SA, Moore CB, et al. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis 2009;49:928- 930.]. Serum liver enzymes should be monitored during therapy. Activity of serum alanine aminotransferase (ALT) greater than 200 U/L in the presence of signs of toxicity may warrant discontinuation of itraconazole until appetite returns and ALT activity returns to <100 U/L [26Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.]. Itraconazole may be restarted at half of the former dose. Ulcerative dermatitis was also observed in 7.5% of dogs receiving itraconazole at 10 mg/kg/d and is associated with elevated itraconazole blood levels. Skin lesions usually resolve rapidly with dose reduction or discontinuation followed by resumption at a lower dose after lesions have resolved [23Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.].
Fluconazole. Fluconazole is often used for treatment of blastomycosis because of its lower cost, and in some cases because of its ocular or cerebrospinal fluid penetration. Response to itraconazole and fluconazole was compared in a retrospective study [27Mazepa AS, Trepanier LA, Foy DS. Retrospective comparison of the efficacy of fluconazole or itraconazole for the treatment of systemic blastomycosis in dogs. J Vet Intern Med 2011;25:440-445.]. Of note is that severity of respiratory disease was greater in dogs treated with itraconazole, while neurologic disease was more common in the fluconazole group. Ninety percent of dogs treated with itraconazole at an average dose of 5 mg/kg/d, for a median time of 4 months, achieved clinical remission, but 18% relapsed. Seventy-five percent of dogs treated with fluconazole 10 mg/kg/d, for six months, responded to therapy, but 22% relapsed. There was a 10% and 25% mortality rate with itraconazole and fluconazole, respectively. While the differences in survival and relapse rates were not statistically significant, they suggest that itraconazole is more effective than fluconazole, which is the case in humans [28Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801-1812.]. The primary indication for fluconazole is where itraconazole cannot be used because of toxicity. Traditionally, fluconazole blood level monitoring has been considered unnecessary because levels were thought to be predictable but based recent findings of a population based pharmacokinetic study in dogs and cats, this dogma should be revisited [29KuKanich K, KuKanich B, Lin Z, et al. Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease. J Vet Pharmacol Ther 2020.].
A potential limitation of fluconazole use is the development of resistance in isolates from patients who have relapsed while receiving fluconazole, which has been documented in humans with histoplasmosis and 1 case in a cat [30Wheat LJ, Connolly P, Smedema M, et al. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clin Infect Dis 2001;33:1910-1913.,31Renschler JS, Norsworthy GD, Rakian RA, et al. Reduced susceptibility to fluconazole in a cat with histoplasmosis. JFMS Open Rep 2017;3:2055116917743364.]. Whether development of resistance was the cause of failure in veterinary patients with blastomycosis has not been studied, but should be considered. Histoplasma isolates that are resistant to fluconazole may also become resistant to voriconazole (unpublished studies at MiraVista Diagnostics) [32Wheat LJ, Connolly P, Smedema M, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother 2006;57:1235-1239.].
Other azoles. Other treatment options include newer azoles such as posaconazole and isavuconazole. They are active in vitro [30Wheat LJ, Connolly P, Smedema M, et al. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clin Infect Dis 2001;33:1910-1913.] and effective in animal models of blastomycosis [33Gonzalez GM, Fothergill AW, Sutton DA, et al. In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi. Med Mycol 2005;43:281-284.,34Sugar AM, Liu XP. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother 1996;40:1314- 1316.,35Sugar AM, Liu XP. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob Agents Chemother 2001;45:601-604.]. However, neither has been adequately studied for treatment of blastomycosis in dogs or cats. There are case reports of patients with CNS blastomycosis treated successfully with voriconazole [36Bakleh M, Aksamit AJ, Tleyjeh IM, et al. Successful treatment of cerebral blastomycosis with voriconazole. Clin Infect Dis 2005;40:e69-71.,37Borgia SM, Fuller JD, Sarabia A, et al. Cerebral blastomycosis: a case series incorporating voriconazole in the treatment regimen. Med Mycol 2006;44:659-664.,38Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 2010;50:797-804.]. These newer azoles are more expensive than itraconazole and might require therapeutic drug monitoring to assure adequate blood levels. Ketoconazole is less effective than other azoles and is not recommended.[39Legendre AM, Selcer BA, Edwards DF, et al. Treatment of canine blastomycosis with amphotericin B and ketoconazole. J Am Vet Med Assoc 1984;184:1249-1254.]
Terbinafine. Blastomyces is susceptible to this allylamine antifungal agent [40Ryder NS. Activity of terbinafine against serious fungal pathogens. Mycoses 1999;42 Suppl 2:115-119.]. Moreover, a pharmacokinetic study in dogs showed that a 30-35 mg/kg dose provided blood concentration above the reported MIC for Blastomyces for approximately 18 hours. Studies to evaluate its effectiveness in blastomycosis are lacking but some veterinarians use it as salvage therapy in patients failing other treatments (personal communications).
Duration of treatment. The optimal duration of therapy is unclear as prospective studies have not been conducted. Plumb recommends two months for fluconazole, but two to three months for itraconazole [41Plumb D. Itraconazole. in Veterinary Drug Handbook, 9th ed. Plumb DC Ed. Pharma Vet Inc. Stockholm, WI;2018.]. Legendre treated dogs with itraconazole for two months and 28% relapsed, concluding that longer treatment was needed [26Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.]. A retrospective study in dogs treated with fluconazole for a median of six months reported 21% of dogs that survived initial treatment relapsed [27Mazepa AS, Trepanier LA, Foy DS. Retrospective comparison of the efficacy of fluconazole or itraconazole for the treatment of systemic blastomycosis in dogs. J Vet Intern Med 2011;25:440-445.]. Relapse rate was 26% in a prospective study of dogs treated with 6 months of fluconazole [42Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.]. Bromel recommends that at least four to six months of itraconazole should be given to reduce the likelihood of relapse [1Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 2005;20:227-232.]. Together these studies suggest that treatment should be continued for at least 6 months and likely longer in most dogs. 6-12 months is recommended in humans [28Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801-1812.].
Adjunctive therapy. In one study, lung infiltrates worsened in the initial week of therapy, attributed to an inflammatory response to antigens released from dying organisms [42Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.]. Fifty percent of dogs with severe lung disease die during the first week of therapy. Dexamethasone (0.25-0.5 mg/kg IV for 2-3 days) may be given to dogs that develop life-threatening respiratory signs [7Legendre AM. Blastomycosis. in Infectious Diseases of the Dog and Cat. 4th ed. Greene CE Ed. St. Louis, MO: Elsevier; 2012.].
Antigen monitoring. A prospective study assessed antigen clearance during treatment with fluconazole [42Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.]. The antigen concentration decreased during treatment but there was no correlation between residual antigen concentration at discontinuation of treatment and the occurrence of relapse. The authors proposed criteria for stopping treatment: normal physical exam including fundic evaluation, normal or static chest radiographic findings, and to be conservative, a negative urine antigen test. Consultation should be sought if antigen levels are not declining after 3 months of treatment or if treatment must be extended beyond 12 months based on the above criteria.
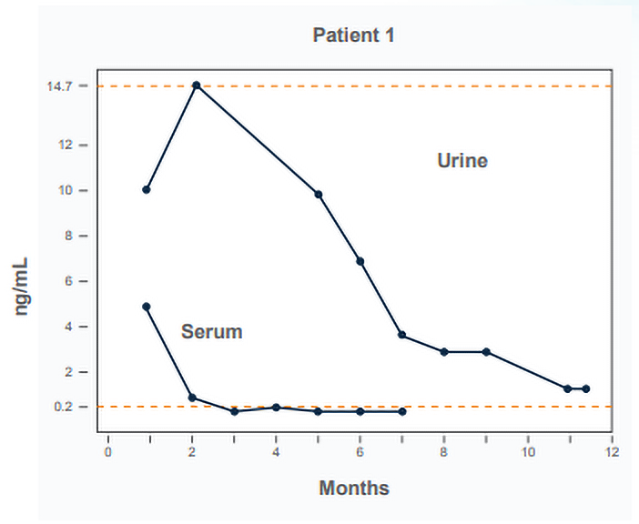
Antigen should be tested in urine in at least 3-month intervals during treatment, at 6 and 12 months after stopping treatment, and any time the clinical findings suggest recurrence. If urine antigen is “Above Limit of Quantification, ALQ” serum should be used for treatment monitoring instead. When the serum antigen is negative, resume monitoring urine antigen until negative.
Failure of the antigen concentration to decline also raises concern about the effectiveness of treatment, which may be caused by inadequate itraconazole blood levels or development of resistance to fluconazole, based upon the experience in histoplasmosis [32Wheat LJ, Connolly P, Smedema M, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother 2006;57:1235-1239.]. If itraconazole levels are subtherapeutic, the dosage should be increased, and blood levels should be rechecked 14-21 days later. Increase in antigen concentration after stopping treatment suggests relapse.
Table 1. Clinical findings in dogs with blastomycosis.[42Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.]
| Finding |
Frequency (%) |
| Tachypnea |
46 |
| Skin lesions |
45 |
| Ocular disease |
42 |
| Fever |
40 |
| Lymphadenopathy, peripheral |
40 |
| Bone/joint disease |
15 |
| Neurologic disease |
5 |
Table 2. Diagnostic performance of antigen and antibody testing for blastomycosis in dogs and cats.
| Test |
Specimen |
Sensitivity (%) |
Specificity (%) |
Reference |
| Blastomyces antigen |
Urine (dog)
Serum (cat) |
100
100 |
95
100 |
16 |
Blastomyces Antibody EIA
BlastomycesAntibody ID
Blastomyces Antibody ID |
Serum (dog)
Serum (dog)
Serum (dog) |
95
68
18 |
95
100
NA |
16
16
15 |
Table 3. What test to order for diagnosis of blastomycosis in dogs and cats
| Endemic |
Primary |
Secondary |
| Blastomycosis |
Blastomyces urine antigen
(code 316) |
Blastomyces IgG antibody-code 330
(canine, no feline IgG)*
Blastomyces FID antibody-code 322
(feline only) |
Secondary tests should be considered if the primary test is negative.
*Most important if the cost for all secondary tests is prohibitive |
Table 4. Treatment recommendations for dogs and cats with blastomycosis.
| Category |
Dose |
Duration |
| Dogs |
Itraconazole 10 mg/day for 3 days then 5 mg/kg/day |
6-12 months |
| Cats |
Itraconazole 10 mg/kg/day (capsule) or 7.5 mg/kg/day (solution) |
6-12 months |
Severe disease* |
Amphotericin B 1.0 mg/kg (dog)^ or 0.5 mg/kg (cat) IV 3 times/week (or EOD) |
Up to 12 mg/kg (cat) or 24 mg/kg (dog) cumulative dose |
Itraconazole as above |
6-12 months |
*Administration of anti-inflammatory doses of corticosteroids may reduce amphotericin B toxicity and inflammatory response to antigens released from Histoplasma yeast
^ This is a starting dose and dogs might tolerate higher doses (2-3 mg/kg/dose)
- Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 2005;20:227-232.
- Baumgardner DJ, Paretsky DP, Yopp AC. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Med Vet Mycol 1995;33:171-176.
- Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213:658-664.
- Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201:1754-1759.
- Selby LA, Becker SV, Hayes HW, Jr. Epidemiologic risk factors associated with canine systemic mycoses. Am J Epidemiol 1981;113:133-139.
- Davies JL, Epp T, Burgess HJ. Prevalence and geographic distribution of canine and feline blastomycosis in the Canadian prairies. Can Vet J 2013;54:753-760.
- Legendre AM. Blastomycosis. in Infectious Diseases of the Dog and Cat. 4th ed. Greene CE Ed. St. Louis, MO: Elsevier; 2012.
- Bloom JD, Hamor RE, Gerding PA, Jr. Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985-1993). J Am Vet Med Assoc 1996;209:1271-1274.
- Hendrix DV, Rohrbach BW, Bochsler PN, et al. Comparison of histologic lesions of endophthalmitis induced by Blastomyces dermatitidis in untreated and treated dogs: 36 cases (1986-2001). J Am Vet Med Assoc 2004;224:1317-1322.
- Gilor C, Graves TK, Barger AM, et al. Clinical aspects of natural infection with Blastomyces dermatitidis in cats: 8 cases (1991-2005). J Am Vet Med Assoc 2006;229:96-99.
- Breider MA, Walker TL, Legendre AM, et al. Blastomycosis in cats: five cases (1979-1986). J Am Vet Med Assoc 1988;193:570-572.
- Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc 1996;32:380-391.
- Buyukmihci N. Ocular lesions of blastomycosis in the dog. J Am Vet Med Assoc 1982;180:426-431.
- Durkin M, Witt J, Lemonte A, et al. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol 2004;42:4873-4875.
- Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 2008;22:839-843.
- Mourning AC, Patterson EE, Kirsch EJ, et al. Evaluation of an enzyme immunoassay for antibodies to a recombinant Blastomyces adhesin-1 repeat antigen as an aid in the diagnosis of blastomycosis in dogs. J Am Vet Med Assoc 2015;247:1133-1138.
- Bialek R, Cirera AC, Herrmann T, et al. Nested PCR assays for detection of Blastomyces dermatitidis DNA in paraffin-embedded canine tissue. J Clin Microbiol 2003;41:205-208.
- Burgess JW, Schwan WR, Volk TJ. PCR-based detection of DNA from the human pathogen Blastomyces dermatitidis from natural soil samples. Med Mycol 2006;44:741-748.
- Babady NE, Buckwalter SP, Hall L, et al. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011;49:3204-3208.
- Sidamonidze K, Peck MK, Perez M, et al. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J Clin Microbiol 2012;50:1783-1786.
- Krawiec DR, McKiernan BC, Twardock AR, et al. Use of an amphotericin B lipid complex for treatment of blastomycosis in dogs. J Am Vet Med Assoc 1996;209:2073-2075.
- Sykes J. Canine and Feline Infectious Disease. St. Louis, MO: Elsevier Saunders; 2019.
- Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats J Am Anim Hosp Assoc 2018;54:195-200.
- Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009;53:24-34.
- Lestner JM, Roberts SA, Moore CB, et al. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis 2009;49:928- 930.
- Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.
- Mazepa AS, Trepanier LA, Foy DS. Retrospective comparison of the efficacy of fluconazole or itraconazole for the treatment of systemic blastomycosis in dogs. J Vet Intern Med 2011;25:440-445.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801-1812.
- KuKanich K, KuKanich B, Lin Z, et al. Clinical pharmacokinetics and outcomes of oral fluconazole therapy in dogs and cats with naturally occurring fungal disease. J Vet Pharmacol Ther 2020.
- Wheat LJ, Connolly P, Smedema M, et al. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clin Infect Dis 2001;33:1910-1913.
- Renschler JS, Norsworthy GD, Rakian RA, et al. Reduced susceptibility to fluconazole in a cat with histoplasmosis JFMS Open Rep 2017;3:2055116917743364.
- Wheat LJ, Connolly P, Smedema M, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother 2006;57:1235-1239.
- Gonzalez GM, Fothergill AW, Sutton DA, et al. In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi. Med Mycol 2005;43:281-284.
- Sugar AM, Liu XP. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother 1996;40:1314- 1316.
- Sugar AM, Liu XP. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob Agents Chemother 2001;45:601-604.
- Bakleh M, Aksamit AJ, Tleyjeh IM, et al. Successful treatment of cerebral blastomycosis with voriconazole. Clin Infect Dis 2005;40:e69-71.
- Borgia SM, Fuller JD, Sarabia A, et al. Cerebral blastomycosis: a case series incorporating voriconazole in the treatment regimen. Med Mycol 2006;44:659-664.
- Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 2010;50:797-804.
- Legendre AM, Selcer BA, Edwards DF, et al. Treatment of canine blastomycosis with amphotericin B and ketoconazole. J Am Vet Med Assoc 1984;184:1249-1254.
- Ryder NS. Activity of terbinafine against serious fungal pathogens. Mycoses 1999;42 Suppl 2:115-119.
- Plumb D. Itraconazole. in Veterinary Drug Handbook, 9th ed. Plumb DC Ed. Pharma Vet Inc. Stockholm, WI;2018.
- Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.



