Treatment of Blastomycosis in Dogs and Cats
Summary
- Generic FDA approved itraconazole is the treatment of choice, 5 mg/kg twice daily for 3 days and then once daily (do not combine with terbinafine, unnecessary). See dosing for cats, differs from dogs.
- Itraconazole should be administered with food.
- Serum should be obtained for trough (just before dosage) itraconazole blood levels 2 weeks after starting treatment (do not combine with terbinafine-falsely elevated itraconazole levels in bioassay).
- Itraconazole dosage should be adjusted to achieve trough levels of 2-7 mcg/mL.
- Treatment could be stopped if (a) at least 6 months treatment, (b) clinical findings resolved, (c) imaging abnormalities resolved, (d) antigen levels urine are negative (or serum if they are higher than urine).
- If criteria for stopping treatment or not met continue treatment for another 3 months and reassess criteria for stopping treatment.
- Antigen levels in urine (or serum if they are higher than urine) should be tested at 6 and 12 months after stopping or if suspect relapse.
- Hepatic enzymes and bilirubin should be measured at baseline, periodically during treatment and if hepatotoxicity is suspected.
- Obtain consultation if these criteria have not been met by 12 months or you have other questions.
Introduction
Several studies have evaluated fluconazole and itraconazole for treatment of blastomycosis in dogs and humans, but none have determined the best treatment or duration of treatment. Only two studies in dogs were prospective (Table 1). The itraconazole study evaluated 107 dogs that were treated for only two months and many of the dogs were severely ill, dying during the first week of treatment [1
Treatment
FDA approved itraconazole is recommended for treatment of blastomycosis in dogs and humans [4
Life-Threatening Illness
Euthanasia may be avoided by an aggressive approach to life-threatening illness. Lipid or liposomal encapsulated (Abelcet® or Ambisome®) is recommended at a dosage of 1.0 mg/kg, three times weekly (or EOD) by intravenous infusion, over 4-6 hours for a cumulative dose up to 12 mg/kg (cat) or 24 mg/kg (dog) (Table 2). Low dose corticosteroids may reduce systemic toxicity caused by amphotericin B (fever, chills, malaise) and worsening of clinical signs related to inflammatory reaction to antigen released by dying fungal organisms. Renal function, electrolytes, and potassium levels should be monitored during treatment. Respiratory failure is the cause of death in most cases and supplemental oxygen is appropriate. Reasons that amphotericin B is more effective than itraconazole include immediate achievement of therapeutic blood levels and its fungicidal mode of action: itraconazole requires up to a week to reach therapeutic levels and is fungistatic.
Treatment Ocular Disease
The best treatment for ocular blastomycosis is uncertain. Some veterinary ophthalmologists prefer fluconazole because of higher penetration than itraconazole into ocular fluids and tissues [8
Treatment CNS disease
Information on treatment of CNS blastomycosis is not available in veterinary patients. Initial treatment with a lipid or liposomal formulation of amphotericin B followed by fluconazole, itraconazole or voriconazole is recommended in humans [5
Itraconazole penetrates CSF poorly but achieves good concentration in brain tissue [8
Combined Treatment with Terbinafine
Some veterinarians recommend terbinafine in combination with other antifungal agents in dogs failing treatment. In vitro activity has been demonstrated with MICs below 0.39 µg/ml in 90% of strains [16
Role of anti-inflammatory treatment
A retrospective study reported that administration of steroids did not improve survival [19
Formulation
Generic FDA approved itraconazole, compounded FDA approved itraconazole capsules (see below), and brand name Sporanox capsules contain “pelletized” drug coated onto spheres and polyethylene glycol, which improves oral bioavailability. Itraconazole capsules should be administered with food for better absorption (see Sporanox capsule package insert). Sporanox® and Itrafungol® oral solutions contains cyclodextrin which improves bioavailability. Blood levels of itraconazole are similar with FDA approved brand name Sporanox® and generic FDA approved itraconazole [6
Dosage
Itraconazole should be administered 5 mg/kg twice daily for 3 days and then once daily in dogs [1
Small dogs pose challenges for use of 100 mg capsules. Alternatives include Sporanox solution (more expensive than generic itraconazole) and compounded pelletized itraconazole capsules. At least two veterinary pharmacies provide that service: Pet Health (623-214-2791); Pet Apothecary (414-247-8633). Prices for FDA approved itraconazole are presented in Table 4 but should be verified for your location. Alternatively, some veterinarians prescribe 100 mg generic FDA approved capsules and instruct the pet owners to empty the capsules onto a hard surface and divide the pellets into roughly equal size piles in dosages appropriate for the weight of the dog. They instruct the owners to coat the pellets onto peanut butter for administration. Finally, findings of a pharmacokinetic study support the use of 100 mg itraconazole capsules once every other day in cats [21
Therapeutic drug level monitoring
It’s important to obtain serum for trough (just before next dose) itraconazole blood level at the end of 2 weeks in dogs and 3 weeks in cats to verify than concentrations are between 2-7 mcg/mL. A dosage change is recommended if concentrations are outside that range. Blood levels should be determined again following a dosage change to verify levels between 2-7 mcg/mL. Itraconazole blood level testing measured by bioassay is offered at MiraVista Diagnostics.
Duration
At least 6 months of itraconazole is recommended in humans [5
Toxicity and Drug Interactions
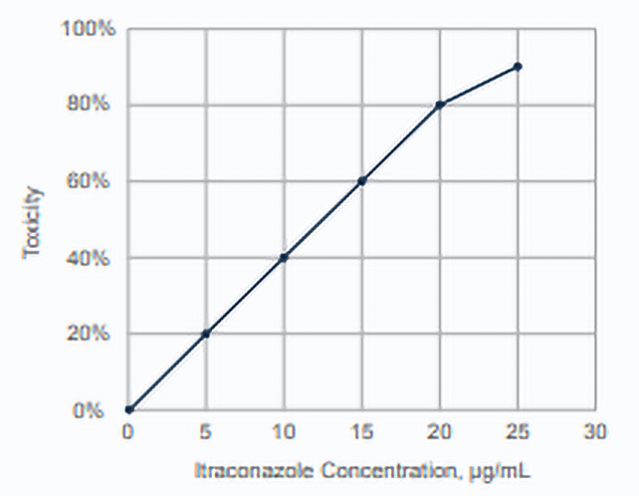
Toxicities are usually caused by high blood levels. A human study reported 31% of patients with levels below 17 µg/mL experienced toxicity compared to 86% with levels above 17.1 mcg/mL. Toxicity occurred in 20 to 40% of patients with levels between 5 and 10 mcg/mL and may occur with levels between 2-7 mcg/mL. Levels above 7 mcg/mL are unnecessary and dose reduction decreases toxicity and cost.
The most common toxicities observed in humans were fluid retention (21%) and gastrointestinal intolerance (21%) [22
Toxicities in dogs resemble those in humans. Median ALT levels were higher in dogs receiving 5 mg/kg twice daily (84 IU/L) than once daily (35 IU/L). ALT and ALP elevation in the absence of clinical findings of hepatotoxicity can be managed by dose reduction or discontinuation until they have resolved, after which itraconazole could be resumed at a lower dosage [1
Most toxicities resolve within a week or two after stopping or reducing the dose of itraconazole. Drug levels should be rechecked 2 weeks after resuming itraconazole to verify the level is therapeutic but nontoxic. Itraconazole can be resumed at a lower dose in dogs with clinical evidence of hepatotoxicity once clinical findings have resolved and ALT and ALP levels declined [1
Itraconazole inhibits cytochrome P450 3A4 isoenzymes and may increase levels of drugs cleared by that mechanism. Serious drug interactions, including death may occur when itraconazole is used with certain drugs that are cleared by P450 CYP3A4 [24
Inducers of CYP3A4, such as phenobarbital, accelerate metabolism of itraconazole and may cause treatment failure because of subtherapeutic blood levels. Drugs that decrease gastric acid secretion reduce itraconazole blood levels. Review potential drug interactions in patients receiving medications that affect or are affected by hepatic metabolism.
Antigen monitoring
A prospective study assessed antigen clearance during treatment with fluconazole [2
Antigen should be tested in urine at least every 3 months during treatment, at 6 and 12 months after stopping treatment, and any time the clinical findings suggest recurrence. And if the urine antigen is “Above Limit of Quantification, ALQ” serum should be used for treatment monitoring instead. When the serum antigen is negative resume monitoring urine antigen until negative.
Alternatives to Itraconazole
Fluconazole is not recommended as a first-line treatment because in vitro activity against Blastomyces is inferior to that of itraconazole (Table 3). It should be noted that a retrospective study failed to detect a significant difference in survival or relapse rates between dogs with blastomycosis treated with itraconazole or fluconazole [3
Cost for itraconazole treatment
Prices for FDA approved itraconazole are presented in Table 4 but should be verified for your location.
When to Stop Treatment
Treat for at least 6 months and until (1) clinical findings have resolved, (2) radiographic findings have resolved or improved significantly and are thought to represent residual scarring, and (3) antigen test is negative. If these criteria are not met, continue treatment for another 3 months and reevaluate. Obtain a consultation if the antigen concentration has not decreased after 3 months of treatment or remains positive after 12 months of treatment.
| Drug | Dose | Duration | Death | Relapse | Reference |
|---|---|---|---|---|---|
| Itraconazole Prospective (n=91)1 | 5 or 10 mg/kg/d | 2 months | 25% (most occurred in the first week) | 39% | 1 |
| Itraconazole Retrospective (n=31)3 | 4.6-10.8 mg/kg/d | Median 138 days | 10% | 18% | 3 |
| Fluconazole Prospective (n= 21)2 | 10 mg/kg/d | Most received 6 months | 0%* | 26% | 2 |
| Fluconazole Retrospective (n=36)3 | 2.9-19 mg/kg/d | Median 183 days | 25% | 22% | 3 |
| *Only the survivors were enrolled in the study. | |||||
| Category | Dose | Duration |
|---|---|---|
| Mild-Moderate | Itraconazole: 10 mg/kg/day for 3 days then 5 mg/kg/day (dog) 10 mg/kg/day (capsule cat) 7.5 mg/kg/day (solution cat) |
6-12 months |
| Severe disease* | Amphotericin B#: 0.5 mg/kg (cat) or 1.0 mg/kg (dog) IV 3 times a week or EOD |
Up to 12 mg/kg (cat) or 24 mg/kg (dog) cumulative dose |
| Itraconazole as above | 6-12 months |
#Abelcet® or Ambisome®
| Itra | Flu | Vori | Posa | References | |
|---|---|---|---|---|---|
| 0.125 | 28.2 | 0.250 | 0.06 | 25,31-33 | |
| Italics = median or geometric mean MIC, others are MIC90 | |||||
| 20 kg dog | 20 kg dog | 5 kg cat | 20 kg dog | 5 kg cat | 5 kg cat | 5 kg cat | |
|---|---|---|---|---|---|---|---|
| Medication | Generic, Itraconazole tablet 100 mg | Sporanox, Itraconazole tablet 100 mg | Sporanox, solution 10 mg/ml | Generic, Fluconazole tablet 200 mg | Generic, Fluconazole solution 40 mg/ml | ItraFungol, solution 10 mg/ml | Compounded Itraconazole Capsule 25 mg |
| Costco | $39.36 | $841.30 | $259.87 | $66.88 | $45.41 | ||
| CVS | $89.84 | $891.54 | $277.37 | $142.02 | $77.78 | ||
| Walmart | $53.13 | $889.84 | $274.60 | $82.46 | $60.97 | ||
| Chewy | Pricing unique to Chewy or veterinary compounding pharmacies | $114.72 | |||||
| Compounding Pharmacy | $72.00 – $260.00 | ||||||
| Dose | 5 mg/kg/d | 5 mg/kg/d | 7.5 mg/kg/d | 20 mg/kg/d | 20 mg/kg/d | 7.5 mg/kg/d | 10 mg/kg/d |
| Lowest cost | $39.36 Costco |
$841.30 Costco |
$259.87 Costco |
$82.46 Costco |
$45.41 Costco |
$114.72 Chewy |
$72.00 compounding pharmacy |
- Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365-371.
- Foy DS, Trepanier LA, Kirsch EJ, et al. Serum and urine Blastomyces antigen concentrations as markers of clinical remission in dogs treated for systemic blastomycosis. J Vet Intern Med 2014;28:305-310.
- Mazepa AS, Trepanier LA, Foy DS. Retrospective comparison of the efficacy of fluconazole or itraconazole for the treatment of systemic blastomycosis in dogs. J Vet Intern Med 2011;25:440-445.
- Sykes JE and Merkel LK. Blastomycosis in Canine and Feline Infectious Disease. Sykes JE Ed. St. Louis, MO. Elsevier Saunders; 2019.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801-1812.
- Renschler J, Albers A, Sinclair-Mackling H, et al. Comparison of Compounded, Generic, and Innovator-Formulated Itraconazole in Dogs and Cats. J Am Anim Hosp Assoc 2018;54:195-200.
- Mawby DI, Whittemore JC, Genger S, et al. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med 2014;28:72-77.
- Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014;27:68-88.
- Hodges RD, Legendre AM, Adams LG, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med 1994;8:409-413.
- Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract 2005;20:227-232.
- Bariola JR, Perry P, Pappas PG, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 2010;50:797-804.
- Haynes RR, Connolly PA, Durkin MM, et al. Antifungal therapy for central nervous system histoplasmosis, using a newly developed intracranial model of infection. J Infect Dis 2002;185:1830-1832.
- Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807-825.
- Sorensen KN, Sobel RA, Clemons KV, et al. Comparison of fluconazole and itraconazole in a rabbit model of coccidioidal meningitis.Antimicrob Agents Chemother 2000;44:1512-1517.
- Tucker RM, Denning DW, Dupont B, et al. Itraconazole therapy for chronic coccidioidal meningitis. Ann Intern Med 1990;112:108-112.
- Shadomy S, Espinel-Ingroff A, Gebhart RJ. In-vitro studies with SF 86-327, a new orally active allylamine derivative. Sabouraudia 1985;23:125-132.
- Sakai MR, May ER, Imerman PM, et al. Terbinafine pharmacokinetics after single dose oral administration in the dog. Vet Derm 2011;22:528-534.
- Hay RJ. Therapeutic potential of terbinafine in subcutaneous and systemic mycoses. Br J Dermatol 1999;141 Suppl 56:36-40.
- Walton RAL, Wey A, Hall KE. A retrospective study of anti-inflammatory use in dogs with pulmonary blastomycosis: 139 cases (2002-2012). J Vet Emerg Crit Care 2017;27:439-443.
- Hough CL. Steroids for acute respiratory distress syndrome? Clin Chest Med 2014;35:781-795.
- Middleton SM, Kubier A, Dirikolu L, et al. Alternate-day dosing of itraconazole in healthy adult cats. J Vet Pharmacol Ther 2016;39:27-31.
- Lestner JM, Roberts SA, Moore CB, et al. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis 2009;49:928-930.
- Plumb D. Itraconazole. In Veterinary Drug Handbook, 9th ed. Stockholm, WI. Pharma Vet Inc.; 2018.
- Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 2000;38:41-57.
- Sugar AM, Liu XP. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother 1996;40:1314-1316.
- Connolly P, Wheat J, Schnizlein-Bick C, et al. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob Agents Chemother 1999;43:322-328.
- Connolly P, Wheat LJ, Schnizlein-Bick C, et al. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis following pulmonary challenge in immunocompromised mice. Antimicrob Agents Chemother 2000;44:2604-2608.
- Proia LA, Harnisch DO. Successful use of posaconazole for treatment of blastomycosis. Antimicrob Agents Chemother 2012;56:4029.
- Day SR, Weiss DB, Hazen KC, et al. Successful treatment of osseous blastomycosis without pulmonary or disseminated disease and review of the literature. Diagn Microbiol Infect Dis 2014;79:242-244.
- Hussaini SMQ, Madut D, Tong BC, et al. Pulmonary blastomycosis presenting as primary lung cancer. BMC Infect Dis 2018;18:336.
- Chapman SW, Rogers PD, Rinaldi MG, et al. Susceptibilities of clinical and laboratory isolates of Blastomyces dermatitidis to ketoconazole, itraconazole, and fluconazole. Antimicrob Agents Chemother 1998;42:978-980.
- Li RK, Ciblak MA, Nordoff N, et al. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob Agents Chemother 2000;44:1734-1736.
- Wiederhold NP, Patterson HP, Tran BH, et al. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J Antimicrob Chemother 2018;73:404-408.