Anti-Histoplasma Antibody Testing Assists with Clinical Decision Making in the Diagnosis and Management of Feline Histoplasmosis
MVISTA® Histoplasma Feline IgG Antibody EIA
CASE PRESENTATION
A 5-year-old DSH from south Texas had been treated two years prior for chronic diarrhea due to histoplasmosis. At that time, Histoplasma capsulatum yeasts were identified on histopathology. The DVM was concerned about a relapse of the disease since similar clinical signs had been recently observed, although he also considered other differential diagnoses as the cause of current diarrhea. Urine Histoplasma antigen test result was very low positive (<0.4 ng/mL). This result has a low positive predictive value, as it is just above the assay cutoff; therefore, such a result may be viewed as “equivocal.” Serum from this cat was submitted for the Histoplasma Feline IgG antibody EIA, and anti-Histoplasma antibody was moderately positive (38 EIA units). The combination of low positive antigen and moderately positive antibody was evidence of ongoing histoplasmosis.
CHALLENGE
-
- MVista® Histoplasma Feline IgG Antibody EIA has fast turnaround time and is semiquantitative (results reported in EIA units [EU] from 10-80+ EU)
- Although IgG could be retained for several years after infection, moderate-high positive antibody values are not typically observed long-term with successful treatment
- When urine antigen results are equivocal low positive or suspected to be false negative, the presence of anti-Histoplasma IgG provides support for active infection
THE MVISTA® ADVANTAGE
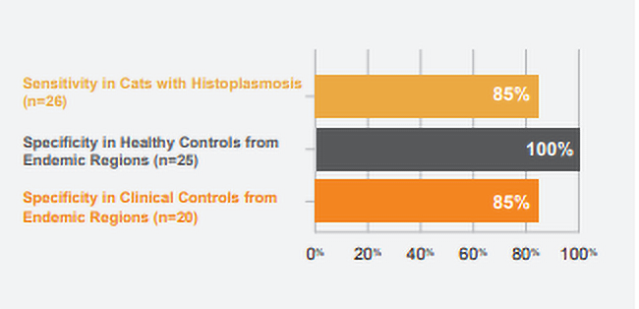
Preliminary unpublished studies using MVista® Histoplasma Feline IgG Antibody EIA
MVISTA® Histoplasma FELINE IGG ANTIBODY EIA
TEST CODE: 328
CLINICAL SIGNIFICANCE: IgG antibodies to Histoplasma antigen appear to be associated with active infection, especially in cats with
moderate to high positive (20 EU or greater) results. Antibodies may also be detected in a small percentage of healthy cats from the endemic area as a result of sub-clinical infection within the last 2 years. IgG may be detected in histoplasmosis cases with falsely-negative antigen results (especially with localized disease or chronic infection) and combined antibody and antigen testing increases the overall sensitivity. Intermediate results (8-9.9 EU) typically reflect either rising or falling IgG, and retesting the patient in several weeks may be beneficial.
SPECIMEN COLLECTION
- Serum: Collect serum specimens in a serum separator or red top tube. Allow blood to clot for 30 minutes, then centrifuge. Pipette serum into a plastic screw cap vial for shipment. If plastic screw cap vial is not available, ship in specimen collection tube.
- CSF: Clean transport tube free of preservatives.
MINIMUM SPECIMEN REQUIREMENTS
- Serum, CSF*, Plasma*: 0.25 mL
- *Uncommon Specimens: these specimens have incomplete validation. A “rare specimen” note will appear on the final report.
SPECIMEN STABILITY
- Room Temp: 28 days
- Refrigerated: 6 months
- Frozen: indefinite
TRANSPORT TEMPERATURE: Refrigerated/Frozen
SHIPPING: Place the specimen and test requisition form in a waterproof, sealable specimen bag. Place the specimen bag and cold pack in a styrofoam container in a box. Ship overnight or second day.
TURNAROUND: Testing is performed on Tuesdays & Thursdays. Serum or CSF: Same or Next Day
REFERENCE RANGE: Negative
INTERPRETATIVE INFORMATION
- Negative: <8.0 EU
- Indeterminate: 8.0 – 9.9 EU
- Positive: 10.0 EU – 80.0 EU
- ALQ: >80.0 EU
METHODOLOGY: Semi-Quantitative Indirect Enzyme Immunoassay
LIMITATIONS: ~1/3 of patients with blastomycosis or coccidioidomycosis will exhibit cross reactivity. The reference range and other method performance specifications have not been established for this test in CSF. The test results should be integrated into the clinical context for interpretation.