Antibody Testing for Histoplasma and Blastomyces ─What, When & Where?
At a Glance – Key Points
- The MVista® Histoplasma and Blastomyces Antigen EIA tests are highly sensitive
- There is high cross-reactivity between Histoplasma and Blastomyces antigen
- Urine and serum antigen testing are negative in approximately 5% of cases
- MVista® Histoplasma and Blastomyces IgG Antibody EIA tests are indicated if antigen testing is negative and clinical suspicion remains
- The MVista® Histoplasma and Blastomyces IgG Antibody EIA tests can help differentiate between histoplasmosis and blastomycosis
Case Example
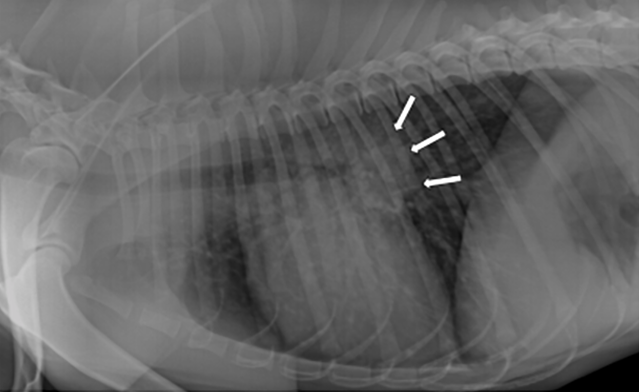
Casey, a 2-year-old, male Labrador retriever was presented for a dry cough and lethargy of a couple weeks duration. Casey lives in southern Kansas on acreage. He has not travelled outside of the state. Physical examination revealed a fever 104.0F and adventitial lung sounds- wheezes. Casey was tachypneic at rest (45 breaths / minute). Routine lab work was unremarkable. Chest radiographs revealed a diffused mostly unstructured interstitial pattern and large tracheobronchial lymph nodes. Based on the full clinical picture, fungal pneumonia was suspected.
Histoplasmosis was considered most likely due to it being the most common systemic mycosis in Kansas. Urine and serum were negative for antigen by the MVista® Histoplasma Antigen Quantitative EIA (test code 310). Due to continued high suspicion for fungal pneumonia, serum was submitted for Histoplasma antibody testing by the MVista® Histoplasma Canine IgG Antibody EIA (test code 327) and Coccidioides antibody testing by MVista® Coccidioides Canine IgG Antibody EIA (test code 329). Anti-Coccidioides IgG antibody concentrations were low (14 EU; Reference Interval <8) while anti-Histoplasma IgG antibody concentrations were at high (>80 EU; Reference interval <8). A small amount of cross-reactivity is expected between the 2 organisms and the significantly higher concentration of anti-Histoplasma antibodies is indicative of histoplasmosis.
Figure 1: Lateral thoracic radiograph showing a diffuse mostly unstructured interstitial pattern and tracheobronchial lymphadenopathy.
Discussion
MVista® IgG antibody testing should be considered when antigen testing is negative, and suspicion remains.
Casey’s case is a good example of when to test for anti-Histoplasma antibodies. When urine is tested for antigen with the MVista® Histo Antigen EIA it is highly sensitive (89% dog and 94% cat) for histoplasmosis [1
MiraVista Diagnostics offers antibody testing by immunodiffusion (ID) and by enzyme immunoassay (EIA). The antibody EIA tests were developed by, and are only available from, MiraVista Diagnostics. The MVista® Blastomyces IgG Antibody EIA has been shown to be far superior to Blastomyces antibody ID testing in dogs [7
MVista® IgG antibody testing can help differentiate between different endemic mycoses.
Like Casey, the antibody EIA tests can help differentiate between different endemic mycoses that potentially crossreact with antigen testing. This would be most common between Histoplasma and Blastomyces, but lesser crossreactivity does occur between Coccidioides and Histoplasma. As in Casey’s case, with minimal cross-reactivity, the antibody concentration is highest for the infecting organism, Histoplasma. In contrast to antibody EIA testing the Histoplasma and Blastomyces Antigen EIA tests are highly cross-reactive, precluding the need to test for both antigens in the same patient.
REFERENCES:
- Rothenburg L, Hanzlicek AS, Payton ME. A monoclonal antibody-based urine Histoplasma antigen enzyme immunoassay (IMMY(R)) for the diagnosis of histoplasmosis in cats. J Vet Intern Med 2019;33:603-610.
- Cunningham L, Cook A, Hanzlicek A, et al. Sensitivity and Specificity of Histoplasma Antigen Detection by Enzyme Immunoassay. J Am Anim Hosp Assoc 2015;51:306-310.
- Cook AK, Cunningham LY, Cowell AK, et al. Clinical evaluation of urine Histoplasma capsulatum antigen measurement in cats with suspected disseminated histoplasmosis. J Feline Med Surg 2012;14:512-515.
- Hanzlicek AS, Meinkoth JH, Renschler JS, et al. Antigen Concentrations as an Indicator of Clinical Remission and Disease Relapse in Cats with Histoplasmosis. J Vet Intern Med 2016;30:1065-1073.
- Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 2008;22:839-843.
- Richer SM, Smedema ML, Durkin MM, et al. Improved Diagnosis of Acute Pulmonary Histoplasmosis by Combining Antigen and Antibody Detection. Clin Infect Dis 2016;62:896-902.
- Mourning AC, Patterson EE, Kirsch EJ, et al. Evaluation of an enzyme immunoassay for antibodies to a recombinant Blastomyces adhesin-1 repeat antigen as an aid in the diagnosis of blastomycosis in dogs. J Am Vet Med Assoc 2015;247:1133-1138.