Improve Canine Blastomycosis Diagnosis with the Blastomyces Antibody EIA
MVISTA® Blastomyces Canine Antibody lgG EIA
Clinical Spotlight
An 8 year old Pomeranian with a solitary bone lesion had a low positive Blastomyces urine antigen result (0.27 ng/mL). This result is near the cutoff for the assay and the submitting DVM considered the possibility of a false positive result. The patient displayed no other clinical signs of blastomycosis and spent the majority of her time indoors. A bone biopsy yielded a non-diagnostic result. The MVista® Blastomyces Canine Antibody lgG EIA result was moderately positive (25 units), indicating recent exposure or active infection with Blastomyces. By combining the positive results in both antigen and antibody tests, the DVM made a presumptive diagnosis of blastomycosis and successfully treated the dog with itraconazole.
Evaluation of an Enzyme Immunoassay for Antibodies to a Recombinant Blastomyces Adhesin-1 Repeat Antigen as an Aid in the Diagnosis of Blastomycosis in Dogs
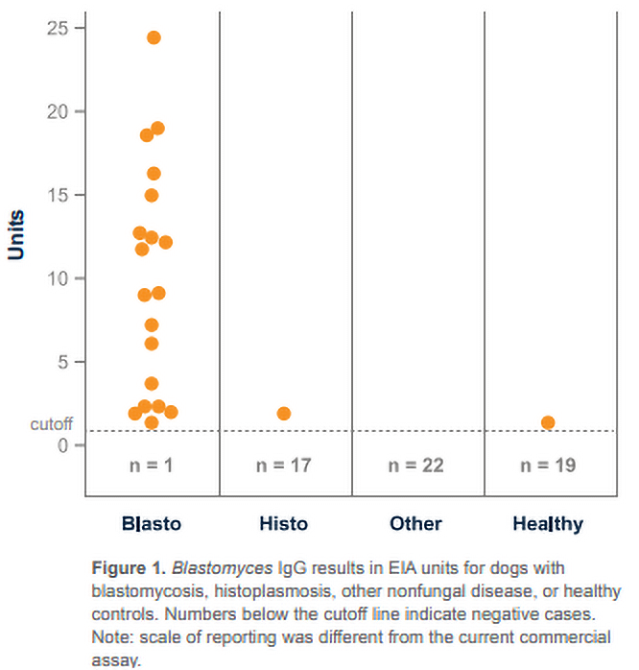
A 2015 study published in JAVMA [1
Current Challenge
Although the sensitivity of the MVista® Blastomyces Antigen EIA in dogs is high [2
The MVista® Advantage
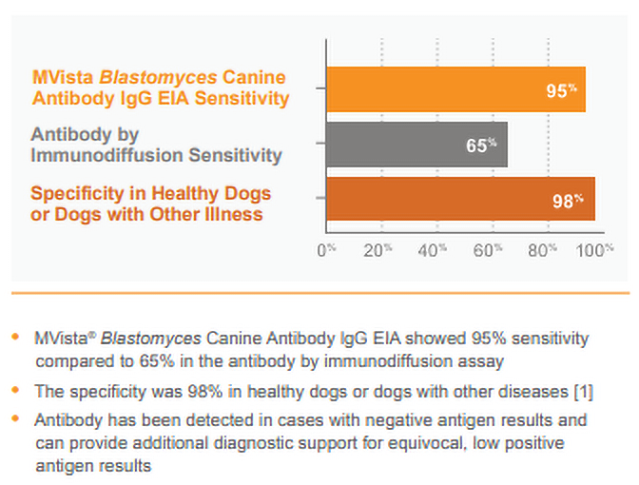
MVista® Blastomyces Antibody lgG EIA offers increased sensitivity compared to immunodiffusion, and high specificity in control dogs.
MVista® Blastomyces Antibody lgG EIA
TEST CODE: 330
CPT CODE: N/A
CLINICAL SIGNIFICANCE: IgG antibodies to Blastomyces antigen appear to be associated with active infection, especially in dogs with moderate to high positive (20 EU or greater) results. Antibodies may also be detected in a small percentage of healthy dogs as a result of sub-clinical infection within the last 2 years; however, results of preliminary studies show high specificity of the assay (>90%) in healthy animals from Blastomyces-endemic areas. IgG may be detected in blastomycosis cases with falsely-negative antigen results (especially with localized disease; e.g., ocular or bone infections) and combined antibody and antigen testing increases the overall sensitivity. Intermediate results (8-9.9 EU) typically reflect either rising or falling IgG, and retesting the patient in several weeks may be beneficial.
SPECIMEN COLLECTION:
- Serum: Collect serum specimens in serum separator or red top tube. Allow blood to clot for 30 minutes, then centrifuge. Pipette serum into a plastic screw cap vial.
- CSF: Sterile transport tube
MINIMUM SPECIMEN REQUIREMENTS:Serum: 0.25ml
SPECIMEN STABILITY:
- Room Temperature: 28 days
- Refrigerated: 6 months
- Frozen: indefinite
SPECIMEN REJECTION: Any specimen type >14 days old other than serum or CSF
TRANSPORT TEMPERATURE: Refrigerated/Frozen
SHIPPING: Ship overnight with cold pack. Monday – Friday delivery.
TURNAROUND:
Serum or CSF:
- Canine: Wednesday & Friday
- Feline: Tuesday & Thursday
REFERENCE RANGE: Antibody Not Detected
INTERPRETATIVE INFORMATION:
- Negative: <8.0 EU
- Indeterminate: 8.0 – 9.9 EU
- Positive: 10.0 EU – 80.0 EU
- ALQ: >80.0 EU
METHODOLOGY: Enzyme Immunoassay
LIMITATIONS:
- May cross-react with other fungi
- The reference range and other method performance specifications have not been established for this test in CSF
- The test results should be integrated into the clinical context for interpretation
REFERENCES:
- Mourning AC, Patterson EE, Kirsch EJ, Renschler JS, Wolf LA, Paris JK, Durkin MM and Wheat LJ. Evaluation of an enzyme immunoassay for antibodies to a recombinant Blastomyces adhesin-1 repeat antigen as an aid in the diagnosis of blastomycosis in dogs. J Am Vet Med Assoc 2015 Nov:247(10):1133-38.
- Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 200 Jul;22(4):839-43.